Influence of Calcium Sources and Concentrations on the Quality and Storage Performance of Peach
Influence of Calcium Sources and Concentrations on the Quality and Storage Performance of Peach
Syed Tanveer Shah* and Muhammad Sajid
Department of Horticulture, The University of Agriculture, Peshawar, KPK, Pakistan
Abstract | A research study was undertaken to study the effect of pre-harvest calcium application on the post harvest life of peach. The research was laid out in Randomized Complete Block (RCB) Design with three factors replicated three times. Peach fruit trees at berry stage were sprayed with three sources of calcium (calcium chloride, calcium nitrate and calcium sulphate) at three calcium concentrations (0.5, 0.75 and 1.0%) stored for 30 days at 10 days interval. The experimental results showed that calcium sources, concentration and storage duration significantly affected all the studied attributes. More fruit firmness (5.57 and 5.69 kg cm2), less Total soluble solids [TSS] (8.57 and 8.26 °Brix) and TSS-acid ratio (12.62 and 11.48) were recorded by the alone application of calcium chloride at 1.0% calcium concentration, respectively. The effect of calcium sources and concentration on the rest of the quality attributes were found non-significant. The means for storage duration showed that freshly harvested fruits resulted in better fruit firmness (5.69 kg cm-2), TSS (8.67 °Brix), ascorbic acid content (6.43 mg 100 g-1), more TSS-acid ratio (0.74), were noted in fruits stored for 10 days. The interaction of Ca-source and Ca-concentration for most of the studied attributes were found significant. It is concluded from the present results that pre-harvest foliar application of CaCl2 (1.0%) at berry sized fruit stage significantly retained the quality attributes for 30 days during storage with an average temperature of 8±2°C and relative humidity of 50%, hence recommended for the grower of Peshawar valley.
Received | June 16, 2017; Accepted | September 16, 2017; Published | October 02, 2017
*Correspondence | Syed Tanveer Shah, The University of Agriculture, Peshawar, KPK, Pakistan; Email: syedtanveer07@aup.edu.pk
Citation | Shah, S.T. and M. Sajid. 2017. Influence of calcium sources and concentrations on the quality and storage performance of peach. Sarhad Journal of Agriculture, 33(4): 532-539.
DOI | http://dx.doi.org/10.17582/journal.sja/2017/33.4.532.539
Keywords | Biochemical attributes, Calcium concentrations, Calcium sources, Peach, Storage duration
Introduction
Among the stone fruits, peach (Prunus persica L.) is one of the important fruit crop of Pakistan. It is a member of family Rosaceae (Chaudhary, 1994), originated from Persia (Iran). The botanical name confirms its origination from Persia (Bassi eand Monet, 2008). History shows that peach was domesticated in Far East geographical origin, way back in 3000 B.C (Li, 1984) as acknowledged in the 19th century (De-Candolle, 1883; Hedrick, 1917; Vavilov, 1951).
Due to less attention, perishability factor and short post harvest life, all horticultural crops faces 17-40% losses especially peach (Rind, 2003). These losses start right from the harvest and thus results in both quality and quantity losses (Armitage and Laushman, 2003). Under normal storage conditions, the life of the peach fruit does not exceed from 3-5 days hence has a short post harvest life. High temperature and low relative humidity during harvesting and marketing are the major factors which reduce the post harvest life of peach fruits (Tonini and Tura, 1998). For getting high yield and good quality fruits, huge efforts are being done but beside these efforts there are many other challenges to be faced during post harvest. As peaches are climacteric in nature, so they pass through high rates of biological activities like respiration and ethylene production. These biological activities lead the fruits towards ripening and detoriation. In order to overcome these losses, different pre and post harvest techniques are being used. These include properly sanitized equipment’s, relative humidity, appropriate temperature and gasses exchange during storage thus enhanced the post harvest life of fruits (Armitage and Laushman, 2003; Young, 2002; Schoellhorn et al., 2003). Among them, calcium is a secondary messenger, is a basic component of cell wall structure that leads to the growth, development and quality of fruit (Kadir, 2004; Kazuhiro et al., 2004). It also minimized the fruit senescence rate (Ferguson, 1984; Gerasopoulos and Drogoudi, 2005), resistance against disease (Elmer et al., 2006; Lanaouskas and Kvikliene, 2006; Tobias et al., 1992; Volpin and Elad, 1991), and reduces other stresses (Yuen, 1993). The effect of calcium on various quality attributes is dependent on its formulation, rate and time of application (Crisosto et al., 1994; Elmer et al., 2006; Kazuhiro et al., 2004). Keeping in view the perishability of peach fruits and the role of calcium in enhancing and retaining the shelf life for a longer period of time, the present study was designed with to find out the effect of various sources and concentration of calcium as pre-harvest foliar spray on quality fruit production of peach cv. Early Grand during storage.
Materials and Methods
The experiment was carried out at Horticultural Research Farm and Postharvest Horticulture Laboratory, The University of Agriculture Peshawar during the year 2014-15, with the objective to find out the best source of calcium and its optimum level to retain the physico-chemical attributes of peach fruits during storage of peach fruit cv. Early Grand.
The experiment was carried out in two phases. During the first phase, peach fruits sprayed with different sources of calcium at various concentrations at plateau stage. The field experiment was conducted by using RCB Design with two factors replicated three times. One control was kept both for calcium sources and concentration. For this purpose, three uniform sized trees were taken randomly for each treatment. During the second phase, the harvested fruits were brought to Post harvest laboratory, Horticulture Department and arranged in three factorial arrangements in RCB Design to study the effect of calcium sources and their concentrations on physico-chemical performance of peach fruit at low temperature (8+2 °C at 50% RH). The details of the factors are given as under.
Factors
Control (for Ca sources and concentration)
Factor A: Calcium Sources (CaC): Calcium chloride (CaCl2), Calcium nitrate (Ca(NO3)2), and Calcium sulphate (CaSO4)
Factor B: Calcium concentrations (CaC): 0.5%, 0.75% and 1.0%
Factor C: Storage duration (SD): 0 days (Fresh harvest), 10 days, 20 days and 30 days
Attributes studied
The data was collected with the following parameters:
Fruits firmness (kg cm-2)
The fruits firmness was measured by using pressure tester (penetrometer). Five fruits were selected from each treatment in each replication. A part of fruit skin of peach was removed with the help of pealer and the pressure tester (hand penetrometer) was slowly penetrated into the internal flesh part of the peach fruit. The reading was recorded, when the inscribed line on the tip reached to the soft tissue of the fruit. After completing the process for all fruits the mean fruit firmness was calculated as reported by Pocharski et al. (2000).
Total Soluble solids (0Brix)
Hand refractometer (Kernco, Instruments Co. Texas) was used to determine the total soluble solid content in peach fruits. The prism of Refractometer was properly cleaned and reading was recorded when a drop of the peach fruit juice was placed on it. Using tissue paper and distilled water, the glass was clean for recording each reading.
Titratable Acidity (%)
It was found by neutralization reaction as described in AOAC (1990).
TSS-acid ratio
TSS-acid ratio was calculated by dividing the total soluble solids and titratable acidity.
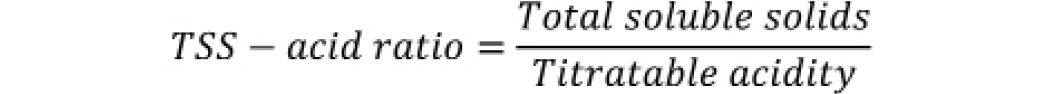
Ascorbic Acid (mg 100g-1)
Titration method (Redox titration) as described by AOAC (1990) was used to calculate the ascorbic acid (%) content of peach.
Statistical analysis
The data recorded were arranged according to Randomized Complete Block Design and was subjected to Analysis of Variance technique as given by Jan et al. (2009). It was then analyzed using statistical software Statistix 8.1 (Statistix_8 Analytical Software. 2003). In case the data was found significant, Least Significant Difference (LSD) test was applied for mean comparison.
Results
Firmness of peach fruits (kg cm-2)
The data showed that Ca sources (CaS), calcium concentrations (CaC) and storage duration (SD) significantly affected fruit firmness of peach. All the interactions were found non-significant except CaS×CaC and CaC ×SD (Table 1).
The data for Ca-sources showed that firmer fruits (5.57 kg cm-2) were obtained from peach plants supplied with calcium chloride (CaCl2) source of calcium. The lowest firmness (5.24 kg cm-2) was observed in peach fruits trees treated with calcium nitrate [Ca(NO3)2].
More firm fruits (5.69 kg cm-2) were taken from peach plants supplied with calcium (Ca) at 1%, followed by fruit firmness (5.27 and 5.20 kg cm-2) was obtained from peach trees sprayed with Ca at 0.75 and 0.5%
Table 1: Fruit firmness (kg cm-2), total soluble solids (°Brix), titratable acidity (%), TSS-acid ratio and ascorbic acid of peach as affected by calcium sources and concentration during storage
| Calcium Sources (CaS) | Parameters | |||||
| Fruit firmness (kg cm-2) | Total soluble solids (0Brix) | Titratable acidity (%) | TSS-acid ratio | Ascorbic acid (mg 100g-1) | ||
|
CaCl2 |
5.57 a | 8.57 c | 0.74 | 12.62 b | 6.38 | |
|
Ca(NO3)2 |
5.24 b | 9.37 a | 0.72 | 13.79 a | 6.38 | |
|
Ca(SO4)2 |
5.35 b | 9.08 b | 0.73 | 13.01 a | 6.34 | |
| LSD (P≤0.05) | 0.16 | 0.272 | NS | 0.922 | Ns | |
| Ca. Concentrations (CaC) | ||||||
| Control | 5.04 c | 9.42 a | 0.69 | 13.51 a | 6.18 | |
| 0.5 | 5.20 bc | 9.44 a | 0.73 | 14.27 a | 6.32 | |
| 0.75 | 5.27 b | 9.30 a | 0.72 | 13.67 a | 6.38 | |
| 1.0 | 5.69 a | 8.26b | 0.73 | 11.48 b | 6.40 | |
| LSD (P≤0.05) | 0.16 | 0.272 | NS | 0.922 | Ns | |
| Storage duration (SD) | ||||||
| 0 | 5.69 a | 8.67 d | 0.76 a | 11.94 c | 6.40 a | |
| 10 | 5.30 b | 8.90 c | 0.73 ab | 11.92 c | 6.42 a | |
| 20 | 5.09 c | 9.28 b | 0.70 b | 13.46b | 6.33 a | |
| 30 | 4.78 d | 9.99 a | 0.64 c | 15.98 a | 5.94 b | |
| LSD (P≤0.05) | 0.18 | 0.314 | 0.035 | 1.064 | 0.214 | |
| Interactions (LSD at P≤0.05) | ||||||
| CaSxCaC | Fig 1 | Fig 3 | -- | -- | -- | |
| Significance | * | *** | Ns | Ns | Ns | |
| CaSxSt | -- | -- | -- | -- | -- | |
| Significance | Ns | Ns | Ns | Ns | Ns | |
| CaCxSD | Fig 2 | -- | -- | -- | -- | |
| Significance | *** | Ns | Ns | Ns | Ns | |
| CaSxCaCxSD | -- | -- | -- | -- | -- | |
| Significance | NS | Ns | Ns | Ns | Ns | |
Means followed by similar letter(s) in column do not differ significantly from one another; NS = Non-significant and **; * = Significant at 5 and 1% level of probability
respectively. The lowest fruit firmness (5.04 kg cm-2) of peach was noted in control treatment.
The means for storage duration showed that fruit firmness was significantly decreased with increase in storage duration. The highest fruit firmness (5.69 kg cm2) was recorded in freshly harvested fruits, followed by firmness (5.30 kg cm2) of peach fruits stored for 10 days. Fruits of 30th day storage showed the lowest fruit firmness (4.78 kg cm2).
A significant variation was observed between the Ca-source and concentration interaction. The highest fruit firmness (5.86 kg cm2) was observed in peach trees sprayed with 1% CaCl2 solution. The lowest fruit firmness (4.86 kg cm2) was observed from fruits of peach plants fertilized with 0.5% Ca(NO3)2 solution (Figure 1).
The interaction of CaC×SD was also found significant. The data showed that highest fruit firmness (6.08 kg cm2) was observed in freshly harvested peach fruits sprayed with 1% Ca solution. Fruits fertilized with 0.5% Ca solution, kept in storage for 30 days recorded the lowest fruit firmness (4.99 kg cm2) (Figure 2).
Total Soluble Solids (°Brix)
The data for total soluble solids (TSS) showed that calcium sources (CaS), concentration (CaC), storage duration (SD) and CaS×CaC interaction significantly influenced the TSS of peach fruits whereas the rest of the interactions two way and three interactions were found non significant (Table 1).
The data for Ca-sources showed that the highest TSS (9.37 °Brix) content of peach fruits were observed in peach trees treated with Ca(NO3)2, followed by Ca(SO4)2 source of calcium where TSS was recorded as 9.08 °Brix. While, CaCl2 source of calcium recorded the lowest TSS (8.57 °Brix) content of peach fruits.
Concerning the means for the application of various concentrations of calcium, its levels significantly retained the TSS of peach fruits during storage. The peach plants sprayed with 0.5, 0.75 and 1% Ca solution produced fruit with TSS contents of 9.44, 9.30 and 8.26 °Brix respectively. Fruits of control treatment showed a TSS content of 9.42 °Brix.
More TSS (9.99 °Brix) content was recorded in peach fruits kept for 30 days in storage while the lowest total soluble solids content (8.67 °Brix) was recorded in control treatment.
Total soluble solids were significantly decreased at each level of calcium in all the calcium sources. However, fruits treated with 0.5% Ca(NO3)2 solution recorded maximum TSS (9.72 °Brix), while minimum TSS (7.33 °Brix) of peach fruits was observed in fruits of peach plants sprayed with 1% CaCl2 solution (Figure 3).
Titratable acidity (%)
Titratable acidity (TA) was non-significantly affected by Ca-sources, concentration and all the two and three way interactions except storage duration (Table 1).
The highest titratable acidity (0.76%) of peach fruits was recorded in freshly harvested fruits, which was statistically at par with titratable acidity (0.73 %) content of peach fruits stored for 10 days. The minimum TA (0.64%) content of peach fruits was taken from fruits of 30 days storage.
TSS-acid ratio
Calcium sources (CaS), calcium concentration (CaC), storage duration (SD) significantly influenced TSS-acid ratio of peach fruits, while all the two and three way interactions were found non-significant (Table 1).
The data for calcium sources showed that CaCl2 proved to be the best Ca-source in terms of retaining TSS-acid ratio (12.62) of peach fruits. The peach fruits sprayed with Ca(NO3)2 and Ca(SO4)3 as source of calcium did not retain the TSS-acid ratio (13.79 and 13.01), respectively during storage up to acceptable consumer preferences.
Concerning the means for Ca-concentrations, the highest TSS-acid ratio (14.27) was recorded in peach fruits treated with calcium at 0.5% while minimum TSS-acid ratio (11.48) was noted in fruits treated with Ca at 1.0%.
The means for storage duration showed that, more TSS-acid ratio (15.98) was found in peach fruits stored for 30 days. The lowest TSS-acid ratio (11.92) was found in peach fruits stored for 10 days which was statistically at par with TSS-acid ratio (11.94) in peach fruits of control treatment.
Ascorbic acid (mg 100g-1)
storage duration (SD) significantly affected ascorbic acid of peach fruits while Ca-sources (CaS), Ca-concentrations (CaC) and CaS×CaC, CaS×SD, CaC×SD and CaS×CaC×SD interactions had a non significant effect on ascorbic acid content of peach (Table 1).
The highest ascorbic acid content (6.42 mg 100g-1) of peach fruits was found in control treatment. On other side when the peach fruit stored for 30 days had the lowest ascorbic acid (5.94 mg 100g-1) content during storage.
Discussion
The present results showed that 12.90% increase was observed in fruit firmness of peach tree sprayed with 1% calcium concentration (highest calcium concentration) as compared to the other levels of control treatment and other level of calcium. As concerned to Ca sources, so fruits treated by calcium chloride source of calcium significantly increased the fruit firmness by 10.52% as compared to control and other sources of calcium (Table 1).
This retention of fruit firmness by calcium application (sources and levels) might be due to the role of calcium in post-harvest life of fruits and vegetables (Rees, 1975). Calcium strengthens the cell wall by binding the free carboxyl group of polygalactruonate polymers (Rees, 1975), resisting the fruit against the hydrolytic enzyme activity (Wills and Rigney, 1979; Buescher and Hobson, 1982), which significantly retained the fruit firmness of peach. The present results are in accordance with Manganaris et al. (2005), they reported that calcium sources significantly retained fruit firmness in peach as compared to untreated fruits. Similarly golden delicious apples treated with calcium chloride (2 and 4%) significantly retained the fruit firmness of apple for 6 months compared to untreated fruits (Picchion et al., 1998). Furthermore, Senevirathna and Daundasekera (2010) found a positive relationship between tomato fruit firmness and calcium chloride concentration treatment.
The decrease in fruit firmness in storage might be due to the breakdown of insoluble protopectin into soluble pectin (Matto et al., 1975), leading to increased membrane permeability (Oogaki et al., 1990). The increase in cell membrane permeability might be due to hydrolysis of intercellular pectins and reduction in cell turgor pressure. These factors resulted a decrease in tissue rigidity and increase in fruit softening (Pollard, 1974), hence fruit firmness decreased. The exogenous calcium application, cross linked the deastrified pectin chain thereby forms a tighter and firmer structure (Grant et al., 1973).
One of the major quality attribute in most of the fruits is the total soluble solids, which is correlated with the texture and composition (Weibel et al., 2004; Peck et al., 2006). Total soluble solids give a rough estimation of the amount of sugars and other soluble minerals present in the fruit. The percentage of sugars present in soluble solids contents is about 80-85% (Peck et al., 2006). In the current study, a percent increase of 9.02 and 6.59% in total soluble solids and TSS-acid ratio of peach fruits are observed in fruits treated with 1% calcium of calcium chloride source as compared to control and other calcium levels and sources (Table 1). This increase in TSS and TSS-acid ratio might be due to the role of calcium.
As the fruit proceeds to ripening process, the degradation of polysaccharides to simple sugars occurs that might increase TSS content (Naik et al., 1993) of the fruits. Calcium is one of the major nutrients that slowed down the respiration and other metabolic activities of the fruit, which retards the ripening process. Furthermore, reduction in respiration rate resulted to slow down the synthesis and utilization of metabolites that eventually decrease the conversion of carbohydrates to sugars which ultimately lowers the TSS content of fruit (Rohani et al., 1997) which greatly confirmed the present results. The present results are in line with the Cheour et al. (1991), who reported that application of calcium delayed the increase in free sugars of fruits, which were steadily increased in storage. The pre-harvest application of calcium increased the calcium content and retained the fruit TSS content, hence regulated various postharvest changes that led to senescence (Cheour et al., 1990).
Conclusions
Based on the present findings, it is concluded that among the calcium sources, foliar application of calcium chloride retained firmness, TSS and TSS-acid ratio of peach compared with other sources of calcium. A significant variation was also observed among the different calcium concentration, 1.0% calcium solution proved to be the best in terms of fruit firmness, TSS, TSS-acid ratio in peach. The effect of calcium sources and concentrations on quality attributes such as ascorbic acid content, titratable acidity was found non-significant. Therefore, peach trees cv. Early Grand could be foliar sprayed with 1.0% CaCl2 to retain the maximum quality attributes during storage up to 30 days at Temp of 8±2 0C with RH 50%.
Author’s contribution
This research is part of Ph.D experimentation of the principal author and the co-author is the Ph.D supervisor of the principal author.
References
AOAC. 1990. Official Methods of Analysis. Analytical Chemist, 15th Edi. Washington DC, USA.
Armitage, A.M. and J.M. Laushman. 2003. Helianthus annuus L. – Annual Sunflower. In: Specialty Cut Flowers. The production of annuals, perennials, bulbs, and woody plants for fresh and dried cut flowers. Timber Press. Portland, O.R. Pp. 319-330.
Bassi, D. and R. Monet. 2008. Botany and Taxonomy. In: Lyne, D. R. and D. Bassi (ed), The Peach Botany, Production and Uses. CAB International, UK. pp: 1-30. https://doi.org/10.1079/9781845933869.0001
Buescher, R.W. and G.E. Hobson. 1982. Role of calcium and chelating agents in regulating the degradation of tomato fruit tissue by polygalacturonase. J. Food Biol. Chem. 6(3): 147-160. https://doi.org/10.1111/j.1745-4514.1982.tb00682.x
Chaudhary, M.A. 1994. Fruit Crops. In: Bashir, E. and R. Bantel (eds), Horticulture. National Book Foundation. Islamabad. Pp. 476-477.
Cheour, F., C.J. Willemot, Y. Arul, J. Desjardins, P.M. Makhlouf and A. Gosselin, 1990. Effects of foliar application of CaCl2 on postharvest strawberry ripening. J. Am. Soc. Hort. Sci. 115: 789-792.
Cheour, F., C.J. Willemot, Y. Arul, P.M. Makhlouf and Y. Desjardins, 1991. Postharvest response of two strawberry cultivars to foliar application of CaCl2. Hort. Sci. 26: 1186-1188.
Crisosto, C.H., R.S. Johnson, J.G. Luza and G.M. Crisosto. 1994. Irrigation regimes affect fruit soluble solids concentration and rate of water loss of ‘O’Henry’ peaches. Hort. Sci. 29(10): 1169-1171.
DeCandolle, A. 1883. L’origine delle piante coltivate.Fratelli Dumolard, Milan, Italy.
Elmer, P.A.G., T.M. Spiers and P.N. Wood. 2006. Effects of pre-harvest foliar calcium sprays on fruit calcium levels and brown rot of peaches. Crop Prot. 26: 11-18. https://doi.org/10.1016/j.cropro.2006.03.011
Ferguson, L.B. 1984. Calcium in plant senescence and fruit ripening. Plant Cell Environ. 7: 477-489. https://doi.org/10.1111/j.1365-3040.1984.tb01438.x
Gerasopoulos, D. and P.D. Drogoudi. 2005. Summer-pruning and preharvest calcium chloride sprays affect storability and low temperature breakdown incidence in kiwifruit. Postharvest Biol. Technol. 36: 303-308. https://doi.org/10.1016/j.postharvbio.2005.01.005
Grant, G.T., E.R. Morris, D.A. Rees, P.J.C. Smith and D. Thom. 1973. Biological interaction between polysaccharides and divalent cations: The egg box model. FEBS Lett, Kil. 32: 195-198. https://doi.org/10.1016/0014-5793(73)80770-7
Hedrick, U.P. 1917. The Peaches of New York.J.B. Lyon Company Printers, Albany, New York. https://doi.org/10.5962/bhl.title.55218
Jan, M.T., P. Shah, P.A. Hollington, M.J. Khan and Q. Shohail. 2009. Agriculture Research: Design and Analysis. 1st Ed. Dept. of Agronomy, The Uni. of Agric., Peshawar, Pakistan.
Kader, A. 2002. Postharvest technology of horticultural crops. Publication 3311. USA, University of California Agricultural and Natural Resources.
Kadir, S.A. 2004. Fruit quality at harvest of “Jonathan” apple treated with foliarly-applied calcium chloride. J. Plant Nutr. 27(11): 1991-2006. https://doi.org/10.1081/PLN-200030102
Kazuhiro, I., M. Masashi and F. Hiroyuki. 2004. The effect of spraying of calcium to the fruit quality, the quality keeping period and the tree vigor of ‘kousui’ in the green house. Bulletin Saga Prefectural Fruit Tree Exp. Sta. 15: 8-14
Lanaouskas, J. and N. Kvikliene. 2006. Effect of calcium foliar application on some fruit quality characteristics of ‘Sinap Orlovskij’ apple. Agron. Res. 4: 31-36.
Li, Z. 1984. Peach germplasm and breeding in China. Hort. Sci. 19: 348–351.
Manganaris, G.A., M. Vasilakakis, I. Mignani, G. Diamantidis and K. Tzavella-Klonari. 2005. The effect of preharvest calcium sprays on quality attributes, physicochemical aspects of cell wall components and susceptibility to brown rot of peach fruits (Prunus persica L. cv. Andross). Scientia Hort. 107: 43–50 https://doi.org/10.1016/j.scienta.2005.06.005
Matto, A.K., T. Murata, E.B. Pantastico, K. Chactin, K. Ogata and C.T. Phan. 1975. Chemical changes during ripening and senescence. In: Postharvest physiology, handling and utilisation of subtropical fruits and vegetables (Ed. E.B. Pantastico). AVI Publishing Co. Inc. Westport, Connecticut, Pp. 103-127.
Naik, D.M., V.G. Mulekar, C.G. Chandel, B.M. Kapse.1993. Effect of prepackaging on physico-chemical changes in tomato (Lycopersicon esculentum Mill.) during storage. Indian Food Packer.
Oogaki, C., H.G. Wang and H. Gemma.1990. Physiological and biochemical characteristics and keeping qualities of temperate fruits during chilled storage. Acta Hort. 279:541-558. https://doi.org/10.17660/ActaHortic.1990.279.61
Peck, G.M., P.K. Andrews, J.P. Reganold and J.K. Fellman. 2006. Apple orchard productivity and fruit quality under organic, conventional, and integrated management. Hort Sci. 41: 99-107.
Picchion, G.A., A.E. Watada, W.S. Conway, B.D.Whitaker and C.E.Sams, 1998. Postharvest calcium infiltration delays membrane lipid catabolism in apple fruit. J. Agric. Food Chem. 46: 2452-2457. https://doi.org/10.1021/jf971083e
Pocharski, W.J., D. Konopacka and J. Zwierz. 2000. Comparison of Magness-Taylor pressure test with mechanical, nondestructive methods of apple and pear firmness measurements. Int. Agrophy. 14: 311-31.
Pollard, J.E. 1974. Pectinolytic enzyme activity and changes in water potential components association with internal breakdown in McIntosh apples. J. Am. Soc. Hort. Sci. 100: 642-649.
Rees, D.A. 1975. Steriochemistry and binding behaviour of carbohydrate chains. In: W.J. Whelan (Ed.), Biochemistry of Carbohydrates .MTP I, Butterworths, London, Unv. Park Press, Baltimore, Inst. Rev. Sci. Biol. Chem. Ser., p. 5.
Rind, S.Y.M. 2003. National horticultural seminar at NARC. PARC News. 23(1).
Rohani, A., W.A. Nazni, L.V. Ngo, J.Ibrahim and H.L. Lee. 1997. Adulticidal properties of the essential extracts of some Malaysian plants on vector mosquitoes. Trop. Biomed. 14: 5-9.
Schoellhorn, R., E. Emino and E. Alvarez. 2003. Specialty cut flower production guides for Florida: Sunflower. Environ. Hort. Department, FL. Coop. Ext. Serv. Institute of Food and Agricultural Sciences, Uni. of Florida. ENH885. Pp. 1-3.
Senevirathna, P.A.W.A.N.K. and W.A.M. Daundasekera. 2010. Effect of postharvest calcium chloride vacuum infiltration on the shelf life and quality of tomato (cv. ‘Thilina’). Cey. J. Sci. (Bio. Sci.). 39 (1): 35-44
Statistix_8 Analytical Software. 2003. Statistix_ 8 User’s Manual. Tallahassee, Florida: Analytical Software. ISBN 1-881789-06-3.
Tobias, R.B., W.S. Conway, C.E. Sams, K.C. Gross and B.D. Whitaker. 1992. Cell wall composition of calcium-treated apples inoculated with Botrytis cinerea. Phytochem. 32:35-39. https://doi.org/10.1016/0031-9422(92)80102-K
Tonini, G. and E. Tura. 1998. Influence of storage and shelf-life time on rots of peaches and nactarines. Acta Hort. 464: 364–367.
Vavilov, N.I. 1951. The Origin, variation, immunity and breeding of cultivated plants. Selected Writings of N.I. Vavilov. Chronica Botanica Company, Waltham, Massachusetts.
Volpin, H. and Y. Elad. 1991. Influence of calcium nutrition on susceptibility of rose flowers to Botrytis blight. Phytopathol. 81: 1390–1394. https://doi.org/10.1094/Phyto-81-1390
Weibel, F., F. Widmer and A. Husistein. 2004. Comparison of production systems: Integrated and organic apple production. Part III: Inner quality: composition and sensory. Obst-und Weinbau. 140: 10-13.
Wills, R.B.H. and C.J. Rigney. 1979. Effect of calcium on activity of mitchondria and pectin enzymes isolated from tomato fruits. J. Food Bio. Chem. 3:103-110. https://doi.org/10.1111/j.1745-4514.1980.tb00639.x
Young, J. 2002. Field grown cut flower production in Southern Louisiana. M.Sc. Thesis. Louisiana State University. Baton Rouge, LA.
Yuen, M.C. 1993. Postharvest handling of tropical fruits. In: Proceedings of the International Conference on Postharvest Handling of Tropical Fruits. 19–21 July, Chaing Mai, Thailand.
To share on other social networks, click on any share button. What are these?








