Efficacy of Naturally Occurring Chemicals for the Integrated Control of Varroa destructor (Anderson and Trueman) in Honeybee Colonies
Efficacy of Naturally Occurring Chemicals for the Integrated Control of Varroa destructor (Anderson and Trueman) in Honeybee Colonies
Rashid Mahmood1*, Muhammad Abu Bakar2, Muhammad Fahim Raza3, Ziyad Abdul Qadir1 and Muhammad Yahya2
1Honeybee Research Institute, National Agricultural Research Centre, Park Road, Islamabad-45500, Pakistan.
2Department of Entomology, University College of Agriculture, University of Sargodha, Sargodha-40100, Pakistan
3Huazhong Agricultural University, Wuhan, China
ABSTRACT
The Varroa mite, Varroa destructor is a destructive ecto-parasite of honey bee (Apis mellifera L) colonies. The main objective of this study was to examine the role of naturally occurring chemicals against Varroa mites of A. mellifera. Nine colonies of A. mellifera were selected to check the infestation of V. destructor randomly. Treatment was applied once and post-treatment data was recorded after 12, 24, 48 and 72 h after all applications of the tested material through sticky cards placed at the bottom of each colony. The results showed that maximum efficacy (76.05 ± 2.28%) was observed in powdered sugar. It was followed by sulfur dust with efficacy of 53.368 ± 1.61%. In addition, we in vitro experiment, five different essential oils were evaluated against Varroa mites. Maximum mite mortality was observed in eucalyptus oil (96 ± 2.89%) followed by winter green oil (79 ± 2.89%), mustard oil (70 ± 2.1%), neem oil (61 ± 1.83%) and orange oil (54 ± 1.62%).
Article Information
Received 19 February 2020
Revised 13 April 2020
Accepted 05 May 2020
Available online 31 March 2021
Authors’ Contribution
RM helped in layout and treatment application along with review and proof reading of manuscript . MAB designed and conducted the study. MFR helped in data collection and analysis. ZAQ helped in review and data interpretation. MY helped in statistical analysis and preparation of manuscript.
Key words
Honeybee, Varroa mites, Essential oils, Sulfur
DOI: https://dx.doi.org/10.17582/journal.pjz/20200219080258
* Corresponding author: [email protected]
0030-9923/2021/0003-1173 $ 9.00/0
Copyright 2021 Zoological Society of Pakistan
Honeybee colonies of Apis mellifera L. are attacked by insects, mites and several diseases (Mahmood et al., ). Two species of mite Varroa destructor Anderson and Trueman (Varroidae: Acrina) and Tropilaelaps clareae, Delfinado and Baker, (Laelapidae: Acrina) are considered to be the cause of destruction of A. mellifera colonies in Asia (). Varroa mites in eastern honey bee colonies cause little damage but after switching hosts and being dispersed across the world through natural and commercial transportation of honey bee colonies, Varroa has become a major pest of honeybee since 1980. V. destructor is now the most serious pest of honeybee colonies in most parts of the world (; ). Only Australia, New Zealand and the state of Hawaii remain free of this pest ().
Previously it was considered that hemolymph of adult honeybees, sealed brood (Pupa) and larvae is the feed of Varroa mite, but it has been reported recently that Varroa mite feeds on fat bodies of honeybees (Ramsey et al., 2019). Contamination of colony with Varroa leads to decrease in body weight, deformation and even death of A. mellifera (Ritter, 1981; Mosaddeg and Komeyli-Birjond, 1988). Varroa mites cause virus disease in honeybees colony such as chronic bee paralysis virus (CBPV) and acute bee paralysis virus (ABPV) and deformed wing virus (Webster and Delaplane, 2001; Delfinado-Baker, 1984). Varroa may also transmit Serratia marcescens, a bacterium that causes septicemia in bees (Garedew et al., 2002). With the introduction and domestication of Apis mellifera liguistica in Pakistan by Honeybee Research Institute, NARC-PARC, Islamabad, Pakistan in 1977-78, Varroa mite became a serious pest of this newly introduced honeybee and destroyed a large number of colonies (Ahmad, 1988).
During the past few decades, pyrethroids have been used to control Varroa. However, recent reports emphasized that the population of Varroa in Europe, North America and Italy have developed resistance to the synthetic pyrethroids (El-Zemity et al., 2006). For possible Varroa control use of powdered sugar by “Dusting” bees and colonies has been a chemical-free method explored by some researchers (Fakhimzadeh, 2000, 2001a, b; Aliano and Ellis, 2005; Ellis et al., 2009). Retreated use of pesticides has caused severe problems such as bee toxicity, increased probability of disease resistance and retention of their residues in honey and bee wax (Watkins, 1997).
Present study aims at determining the damage caused by this multicultural pest in terms of honey yield, and mortality of adult honey bees. Since application of insecticides is not advised to be directly applied in honey bee colonies, alternate methods of mite control could be adopted.
Materials and methods
The field experiment was conducted during June 2018 at apiary of College of Agriculture, University of Sargodha. Powdered sugar and sulfur were used for testing their efficacy against Varroa mites. Thirty colonies of the A. mellifera were selected and selection was dependent on vigor, number of bees and the infestation of mites randomly to check the infestation of V. destructor. Each colony consisted of eight combs containing a queen, worker bees, and drones.
Pre-treatment data of mite infestation was recorded randomly from selected colonies. For this purpose, 150-160 bees infested with mite were collected randomly (Lee et al., 2010). The data of infestation percentage was recorded by following (Ritter, 1981).
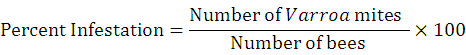
Each of the nine colonies was treated with different doses of powdered sugar (125g. /colony) and sulfur dust at the dose of 1g /frame. Control colonies were left untreated. The experiment was replicated thrice.
To monitor Varroa mite population before and after treatments, a “sticky card” was pushed in all hives bottom prior to each treatment under the wire/wood frame, where falling mites were trapped (Sammataro et al., 2005). Data was recorded after 12, 24, 48, 72 h of treatment and sticky card replaced after each interval. The hive entrances remained open during the experiment and application of dusting was carried out after sunset, when all honeybees had returned to the hives. The application of sulfur dusting was done by pouring the dust on upper side of each frame with dose rate of 1g /frame and the fallen mites were monitored through the sticky card placed at the bottom of hive.
Efficiency percentage of each application of these compounds was determined using the following formula; (Rashid et al., 2012).

Treatment means were compared by LSD all pair wise test comparison at 5% probability levels. The data collected from experiments were statistically analyzed using statistix 8.1 software. Efficacy five different of essential oils was tested against Varroa mites on honey bees. Eucalyptus oil, winter green oil, mustard oil, orange and neem oil were used at the dose of 0.15 ml with concentration of 100 ppm.
Ten selected worker bees of A. mellifera naturally infested with Varroa mites were collected from Apiary for each treatment. The number of Varroa mites on selected bees were also counted carefully and kept in plastic jars (15 cm ht. x 8 cm) for each treatment. A simple cardboard (2 x 2 cm) soaked with 0.15 ml/100 ppm concentration of the test substance was hanged by means of appropriate wire inside the jar. A screen mesh (8cm) was placed 2 cm above bottom of each jar that allowed dead mites to fall through to a sticky paper placed below. The control treatment jars were left untreated. Bees were fed on sugar solution.
Evaluation of the tested materials and techniques were based on the mortality percentage and it was calculated through the modified Abbott’s formula specified by Henderson and Tilton, (1955).
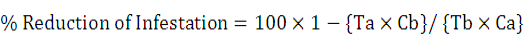
C = % infestation of mites untreated, T = infestation % of mites treated (a = after, b = before treatment).
The mites mortality data was taken after 12, 24 and 48 h of post treatment by counting the fallen mites at the bottom of the jars.
Results and discussion
The results showed that high mortality (76.052±2.28%) was observed when powdered sugar was applied at 125g / colony under field conditions, followed by 53.368±1.6% mortality in sulfur dust administrated at 1g / frame of each colony under the same field conditions 72 h after treatment. Overall, percent mortality was higher in powdered sugar compared to sulfur dust (Fig. 1).
Percent infestation was 21± 0.63 % in the case of powdered sugar and 43± 1.29 % in sulfur dust at 72 h after treatment (Fig. 2).
We found that our treatment protocol did not significantly reduce the total number of mites per colony. Fakhimzadeh (2001a) also did not find complete reduction in colony mite populations after powdered sugar treatment. This may be attributable to the reproduction rates of Varroa mites under differing population pressures. Eguaras et al. (1994) observed that at lower mite densities, the reproductive rate of Varroa increases. Therefore, the mite may be able to compensate for population loss due to dusting by increasing its reproductive rate. Similarly, it may be due to grooming behavior (body cleaning from sugar dust) of honeybees. Stevanovic (2007) found that sugar treatment significantly increased grooming behavior which decreased mite population.
The Varroa mite can be considered as a continuous problem for beekeepers especially since there are various dispersal methods for the Varroa. Chemical control recommendations are being changed frequently as new products are developed and problems with older treatments become evident. The mite is highly resistant to chemical treatments; therefore, even if necessary, the repeated applications of same chemical may be avoided (Abou-Shaara, 2014).
In laboratory experiment, maximum mortality was observed in the case of eucalyptus oil (96.29 %) followed by winter green oil (79.33 %) and orange oil (54.31 %) at 48 h after treatment (Fig. 3). Mite mortality increased after long exposure to essential oils. The control which had no treatment showed only natural mite fall.
Natural products such as essential oils offer a highly desirable alternative to synthetic products (Bakar et al., 2019). Several essential oils have shown acaricidal activity in screening tests. Essential oils are highly volatile terpenes and phenolic compounds, which have an intense aroma (Imdorf et al., 1999). Essential oils are byproducts of the secondary metabolism of certain plants. Plants have evolved the potential use of essential oils to control Varroa mites which was reported by several authors (Hoppe and Ritter, 1997; Bogdanov et al., 1998; Sammataro et al., 1998; Bakar et al., 2019). Treatments with essential oils represent a potentially superior control for Varroa mites. Because of their origin and mode of action, it is possible that such compounds are more easily degraded, more specific and less susceptible to the production of resistance than synthetic pesticides currently used (Bakar et al., 2019). Calderone and Spivak (1995) found that a blend of thymol, eucalyptus oil, menthol and camphor caused average mite mortality of 96.7%.
Conclusion
Sugar powder proved to be best to control Varroa mites and did not affect colony strength of honeybees. The lab bioassays showed that eucalyptus oil was promising as safe natural product for control of Varroa mites. These natural products proved to be harmless to the bees and quite safe to the environment. The use of naturally occurring chemicals may fit well into Integrated Pest Mansagement programs for alternative use with other control measures for the management of Varroa mite and other pests in honeybee colonies.
Acknowledgments
The assistance of Scientific Assistant and Bee Attendants is gratefully acknowledged for collecting mite collection trays.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Abou-Shaara, H.F., 2014. Vet. Med., 59:1-10. https://doi.org/10.17221/7240-VETMED
Ahmad, R., 1988. Prog. Farm., 8: 34-36.
Aliano, N. P. and Ellis, M.D., 2005. J. Apic. Res., 44: 54-57. https://doi.org/10.1080/00218839.2005.11101148
\ֻad, M. and Sohail. M., 2019.Pakistan J. agric. Res., 32: 566-571. https://doi.org/10.17582/journal.pjar/2019/32.4.566.571
Bogdanov, S., Kilchenmann, V., Imdorf, A. and Fluri, P., 1998. Am. Bee J., 138: 610-611.
Calderone, N.W. and Spivak, M., 1995. J. econ. Ent., 88: 1211-1215. https://doi.org/10.1093/jee/88.5.1211
De Jong, D., Roma, D.D.A. and Goncalves, L., 1982. Apidologie, 13: 297-306. https://doi.org/10.1051/apido:19820308
Delfinado-Baker, M., 1984. Int. J. Acarol., 10: 75-80. https://doi.org/10.1080/01647958408683356
Dietemann, V., Pflugfelder, J., Anderson, D., Charrière, J.D., Chejanovsky, N., Dainat, B., De Miranda, J., Delaplane, K., Diller, F., Fuch, S., Gallman, P., Gauthier, L., Imdorf, A., Koeniger, N., Kralj, J., Meikle, W., Pettis, J., Rosenkranz, P., Sammataro, D., Smith, D., Yañez, O. and Neumann. P., 2012. J. Apicult. Res.., 51: 125-132. https://doi.org/10.3896/IBRA.1.51.1.15
Eguaras, M., Marcangeli, J. and Fernandez, N.A., 1994. J. Apicult. Res., 33: 155-159. https://doi.org/10.1080/00218839.1994.11100863
Ellis, A.M., Hayes, G.W. and Ellis, J.D., 2009. J. apicult. Res., 48: 72-76. https://doi.org/10.3896/IBRA.1.48.1.14
El-Zemity, S.R., Rezk, H.A. and Zaitoon. A.A., 2006. J. appl. Sci. Res., 2: 1032-1036.
Fakhimzadeh, K., 2001a. J. Apicult. Res., 40: 105-109. https://doi.org/10.1080/00218839.2001.11101058
Fakhimzadeh, K., 2001b. Apidologie, 32: 139-148. https://doi.org/10.1051/apido:2001119
Fakhimzadeh, K., 2000. Am. Bee J., 140: 487-491.
Garedew, A., Schmolz, E., Schricker, B., Polaczek, B. and Lamprecht, I., 2002. J. Apic. Sci., 46: 73-81.
Henderson, C.F. and Tilton, W., 1955. J. econ. Ent., 48: 157-161. https://doi.org/10.1093/jee/48.2.157
Hoppe, H. and Ritter, W., 1997. Apidologie, 28: 123-127.
Imdorf, A., Bogdanov, S., Ochoa, R.I. and Calderone, N.W., 1999. Apidologie, 30: 209-228. https://doi.org/10.1051/apido:19990210
Lee, K., Moon, R., Burkness, E., Hutchison, W. and Spivak, M., 2010. J. econ. Ent., 103: 1039-1050. https://doi.org/10.1603/EC10037
Mahmood, R., Asad, S., Ahmad, W., Sarwar, G., Rafique, M.K., Islam, N., Qadir, Z.A. and Abiden, Z.U., 2017. Pakistan J. Zool., 49: 9-13. https://doi.org/10.17582/journal.pjz/2017.49.1.8.12
Mosaddeg, M. and Komeyli-Birjond, A., 1988. Harmful mites of honey bee. Shahid Chamran University Publication. (in Persion).
Ramsey, S.D., Ochoa, R., Bauchan, G., Gulbronson, C., Mowery, J.D., Cohen, A., Lim, D., Joklik, J., Cicero, J.M. and Ellis, J.D., 2019. Proc. natl. Acad. Sci., 116: 1792-1801. https://doi.org/10.1073/pnas.1818371116
Rashid, M., Wagchoure, E., Mohsin, A., Raja, S. and Sarwar, G., 2012. J. Anim. Pl. Sci., 22: 72-76.
Ritter, W., 1981. Bee World, 62: 141-153. https://doi.org/10.1080/0005772X.1981.11097838
Rosenkranz, P., Aumeier, P. and Ziegelmann, B., 2010. J. Invertebr. Pathol., 103: 96-119. https://doi.org/10.1016/j.jip.2009.07.016
Sammataro, D., Degrandi-Hoffman, G., Needham, G. and Wardell, G., 1998. Am. Bee J., 138: 681-685.
Sammataro, D., Untalan, P., Guerrero, F. and Finley, J., 2005. Int. J. Acarol., 31: 67-74. https://doi.org/10.1080/01647950508684419
Stevanovic, J., 2007. Ecological-ethological defense mechanisms of Apis mellifera carnica against ectoparasite Varroa destructor on the territory of Serbia. PhD thesis, Belgrade University.
Watkins, M., 1997. Bee World, 78: 15-22. https://doi.org/10.1080/0005772X.1997.11099327
Webster, T.C. and Delaplane, K.S., 2001. Mites of the honey bee. Dadant and Sons, Inc., Hamilton, Illinois. pp. 280.
To share on other social networks, click on any share button. What are these?









