Antagonistic Probioticity of Novel Bacterial Isolates from Pakistan against Fish Pathogen Pseudomonas fluorescens in Labeo rohita Fingerlings
Antagonistic Probioticity of Novel Bacterial Isolates from Pakistan against Fish Pathogen Pseudomonas fluorescens in Labeo rohita Fingerlings
Asma Chaudhary1, Qurat-ul-Ain Ahmad1, Afia Muhammad Akram1, Sadia Roshan2 and Javed Iqbal Qazi3*
1Department of Zoology, Division of Science and Technology, University of Education, Lahore, 54770, Pakistan
2Department of Zoology, University of Gujrat, Gujrat, Pakistan
3Department of Zoology, University of the Punjab, Lahore, 54590, Pakistan
ABSTRACT
The study was endeavoring to investigate in vitro as well as in vivo antibacterial and probiotic potential of Sphingomonas sp. AsCh-P3 (MF543123) and Bacillus aerius AsCh-A7 (MF543123) against fish pathogen Pseudomonas fluorescens. Antagonism by cross streak was observed in ten isolates out of total twenty. Six isolates showed better inhibition by well as well as disk diffusion methods with maximum growth inhibition zones i.e., 15 and 17 mm by Sphingomonas sp. AsCh-P3 and Bacillus aerius AsCh-A7 respectively. Six probiotic bacterial isolates of known enumeration (C.F.U.ml-1) showed growth antagonism for the fish pathogen with inocula comprising of less number of C.F.U.ml-1. Highest survival rates i-e 63.5 and 70.17% were expressed by Sphingomonas sp. AsCh-P3 and B. aerius AsCh-A7 respectively by mixing in formulated fish feed, drying and storing in refrigerator. Mortalities of Labeo rohita fingerlings challenged to P. fluorescens intraperitoneally appeared in a dose dependent manner with symptoms like hemorrhage, swelling of the belly and anus, scale damage etc. The pathogen challenged fish were administered with Sphingomonas sp. AsCh-P3 (group-G1) and B. aerius AsCh-A7 (group-G2) treated feed in different experimental set ups. Highest Relative Percent Survival (80-90%) was recorded for fish receiving probiotic feed prior and after injection (G1b, G2b). Provision of probiotics was evident to alleviate pathogen virulence in fish. The emerging multi-drug resistance and related side effects of conventional therapies have caused researchers to explore alternate strategies, such as probiotics employment for disease control in aquaculture.
Article Information
Received 26 December 2019
Revised 01 March 2020
Accepted 09 March 2020
Available online 14 May 2021
Authors’ Contribution
AC designed and demonstrated the study. QA and AMA analysed experimental data. SR wrote the manuscript. JIQ supervised and guided the trials.
Key words
Probiotics, Virulence, Peudomonas fluorescens, Disease resistance, Antagonistic bacteria to pathogens, Labeo rohita
DOI: https://dx.doi.org/10.17582/journal.pjz/20191226131233
* Corresponding author: qazi.zool@pu.edu.pk
0030-9923/2021/0004-1265 $ 9.00/0
Copyright 2021 Zoological Society of Pakistan
INTRODUCTION
Aquaculture is emerging more speedily compared to other animal food industry. Bacterial diseases are the main hazards to public health and contribute to fish stock fatalities in the industry. To combat diseases, certain measures such as chemotherapy, proper nutrition and immunological manipulation are used.
The indiscriminate consumption of the remedial measures for aquatic animal diseases, including protective chemical additives, veterinary drugs, etc. has supervened the antimicrobial resistance and devastated the bio-environs. There is a dire need to improve microbial control strategies to alleviate the disease incidence and drug resistance (Miranda and Rojas, 2007; Denev et al., 2009; Kolndadacha et al., 2011; Gobinath and Ramanibai, 2012).
Among pseudomonads, Pseudomonas fluorescens and P. putida have been known to be very notorious pathogen species of fresh and brackish water fish. According to Lom and Schubert (1983) P. fluorescens produced open ulcers among the carp. Its signs were scaly erosions; a swollen body cavity, protrusion and reddening of the anus and hyperemia of the swim bladder. This disease received the designation “Septicemic Pseudomonas infection” of the common carp and silver carp.
The exploitation of probiotics as an alternate strategy reinvigorating the therapeutic channel, has been established as positive promoters to improve aquaculture production and gained momentum in recent years (Verschuere et al., 2000; Dahiya et al., 2010, 2012; Sihag and Sharma, 2012). Probiotics are live non-carcinogenic microbes having the potential to reduce pathogen adhesion, boost host’s immune response, break down the indigestible compounds, and improve production of vitamins and enzymes (Gomez-Gil et al., 2000; Abraham and Banerjee, 2007; Welker and Lim, 2011; Divya et al., 2012; Arig et al., 2013; Golic et al., 2017). According to Ishibashi and Yamazaki (2001) probiotics are food or drug containing live microbes improving physiological effects of animal host when ingested.
In aquaculture, feed/water adjusted with probiotics is administered. The usage of feed supplemented with probiotics is an improved technique ensuring the efficacy of the probiotic microorganisms for healthier fish production (Irianto and Austin, 2002). Typically, the probiotics are supplemented to the feed of animals as freeze-dried culture (Nikoskelainen et al., 2001). It is essential to adequately conserve the viability of probiotics throughout its processing, storage and consumption (Linders et al., 1996; Wouters et al., 1998).
Probiotics stimulate the growth and immune response by different types of Gram positive and Gram negative bacteria, yeast, unicellular algae and certain bacteriophages in wide range of fishes when administered in fish feed, water and as disease resistant as was evident in literature such as lactic acid bacteria in larva and Lactobacillus rhamnosus (JCM 1136) in rainbow trout (Panigrahi et al., 2004), Bacillus S11 to shrimp gut (Rengpipat et al., 2000), yeast Zymosan in shrimp (Panigrahi et al., 2004), Different lactobacilli such as Lactobacillus thermophillus, L. plantarum, L. acidophilus, L. casei, L. bulgaricus and L. rhamnosus are used frequenly in fish feed to improve growth and survival as well as immunomodulant to be considered as supplement to vaccines to resist infectious diseases (Nayak, 2010; Esteban et al., 2014; Muñoz-Atienza et al., 2014; Sahoo et al., 2015; Dawood, 2016; Gobi et al., 2016). Probiotics in form of mono culture or multicultures stimulate innate immunity by expressing cytokines, lysozyme and phagocytic activities. They are hypothesized to promote immunoglobulin cells and granulocytes (Nayak, 2010).
The pathogen inhibiting potential of both Gram positive and negative bacterial organisms to overcome infections has been reported in the in vitro and in vivo studies (Itoh et al., 1995; Authira et al., 2011; Gobinath and Ramanibai, 2012; Tabak et al., 2012). The genus Bacillus is considered as a biocontrol agent in feed and water (Abraham and Banerjee, 2007). Bacillus sp. is producing siderophores, hydrogen peroxide, lysozyme, proteases and bacteriocin that inhibit pathogen in the fish intestine (Jones, 2002; Mohideen et al., 2010; Seenivasan et al., 2012). Ghosh et al. (2007) reported antagonistic potential of B. subtilis SG4 from Cirrhinus mrigala against Aeromonas hydrophyla, Psedomonas fluorescens, and Edwardsiella tarda.
Rohu (Labeo rohita) is renowned as the most popular among freshwater fish due to good taste in Pakistan and other Asian countries. It is being reared extensively in fresh water (rivers and ponds) to meet the food requirement of the area. In this study, the mode of transmission of pathogenic P. fluorescens to L. rohita fingerlings have been investigated in vivo. The purpose of the present work was to evaluate the anti-pathogenicity of two bacterial isolates in in vitro and in vivo studies.
MATERIALS AND METHODS
Source of fish pathogen
A virulent strain Pseudomonas fluorescens was isolated on Pseudomonas agar P and Pseudomonas agar F from Labeo rohita having ulcerative disease collected from the Research fish ponds, University of the Punjab, Lahore. The strain was characterized phenotypically and biochemically up to the species level along with the production of the pigment pyocyanin and fluorescein on Pseudomonas P and F base media. The results were compared with Pseudomonas species as described in Bergey’s Manual of Systematic Bacteriology (Uğur et al., 2012)
Screening of potential probiotic bacterial strain
Twenty bacterial isolates (from different homemade yogurt and raw milk samples) as selected from previous study was further processed for evaluation of their antagonistic activities against fish pathogen (Chaudhary and Qazi, 2011).
Probiotic inhibition of the fish pathogen by isolated bacteria
Antagonism of P. fluorescens by the isolated bacteria was evaluated by cross streak technique (Austin et al., 1992). Fish pathogen suspension, prepared in phosphate buffer saline (PBS) (O.D. set at 600 nm at 0.5±0.05) was streaked perpendicularly across already streaked bacterial isolate (with cell densities set as 0.5±0.05 spectrophotometrically) on a solidified nutrient agar plate. After 48 h incubation period at room temperature (RT), antagonism as visible growth interruption of the fish pathogen was observed. On the basis of visible antibiosis, ten isolates were screened against the fish pathogen for subsequent experimentation.
Antagonism assay
Screened ten isolates were further assayed for antagonistic activities against fish pathogen P. fluorescens by filter paper disc and well diffusions methods (Olsson et al., 1992).
The twenty four h young cultures of a pathogen as well as probiotic isolates were prepared in a liquid nutrient medium, incubated at 37 ºC, centrifuged and filter sterilized. Each filter-sterilized inoculated culture fluid (100 µl) was loaded on the sterilized filter paper disc (Whatman No. 1) of 9 mm diameter. Subsequently, the discs were positioned on solidified nutrient medium plates, pre-inoculated with 50 µl of fish pathogen P. fluorescens suspension (cell densities set at 0.5±0.05) by the spread plate method. Growth inhibition zones (GIZ) around each disc were measured after 24-48 h of incubation at RT. For well diffusion method, fish pathogen P. fluorescens suspension was loaded in solidified nutrient medium (at 50ºC in 3% (v/v) ratio) and dispensed in sterile petri plates.
Wells having diameter 9 mm, were punched into nutrient agar plates employing metal borer. Successively, 100 µl of filter sterilized bacterial culture was loaded in each well. The nutrient agar plates with punched wells were incubated at RT (~27-30 ºC) up to 48 h and growth inhibition zones (GIZ) were recorded. Six most efficient bacterial isolates yielded GIZ ≥10 mm in diameter were screened and used for further experimentation.
Antibiosis of probiotic isolate against varying doses of bacterial fish pathogen
To evaluate the antibiotic prospective of probiotics against varying doses of fish pathogen, liquid cultures of probiotic isolates (cell density set as 0.05, 0.1, 0.25, 0.5, at 600 nm wavelength) were made in PBS (pH 8). Probiotic cell suspensions were set at an absorbance of 0.5±0.05 spectrophotometrically in PBS. The probiotic suspensions were enumerated by viable counting and cross streaked perpendicularly across varying cell densities of fish pathogen on solidified nutrient plates. The inoculated plates were then incubated for 24-48 h at RT (~27-30 ºC). Interruption in the growth pattern of the fish pathogen illustrated the antibiosis potential of test probiotic isolates as already established (Austin et al., 1992).
Probiotic supplementation in formulated fish feed
The selected probiotic isolates were cultivated for 24 h in a liquid nutrient medium. Bacterial culture was then centrifuged and suspended in PBS of 0.5 ± 0.05 cell density at 600 nm. Cell suspensions (of 20% v/w) were amalgamated with sterilized formulated fish feed and air dried for three days in the laminar air flow chamber at RT (~27-30 ºC) and kept frozen for a week. Serially diluted processed fish feed samples made in PBS (0.89%) were enumerated by viable counting on solidified nutrient medium. Evaluation of probiotic bacterial survival (%) was made by following calculation.
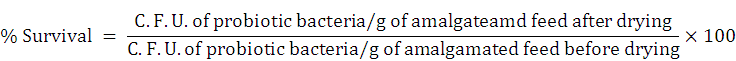
Based on the relatively high antagonistic properties and greater survival potentials, the probiotic isolates Sphingomonas sp. AsCh-P3 and Bacillus aerius AsCh-A7 (later identified through 16S rRNA gene sequencing) were screened for performing inocula optimization in formulated feed.
Optimization of probiotic inocula in formulated feed
To optimize probiotic inocula, feed extract was used. For feed extract preparation, dried feed (20 g) was boiled for 10 minutes in 100 ml distilled water and autoclaved for 15 minutes under standard conditions. The sterilized feed was centrifuged to obtain the clear extract. The probiotic isolates, suspensions of AsCh-P3 and AsCh-A7 in PBS were inoculated with different percent v/v inocula (10, 20, 30) in the sterilized feed extract and incubated at RT (30±0.2oC). Growth was evaluated at constant intervals of twenty four h up to three consecutive days in form of C.F.U.ml-1.
Characterization of probiotic isolates
Cell and colony morphology tests as well as biochemical assays were employed to all the probiotic bacterial isolates for taxonomic categorization (Collins et al., 1995) and subsequent identification. Selected probiotic strains AsCh-P3 and AsCh-A7 were also subjected to molecular characterization through 16S rRNA gene sequencing.
Fresh bacterial culture was used to extract DNA by heating in 50 mM NaOH (45µl) for 5 minutes 95ºC. Subsequently 5 µl of Tris HCl (1M, pH 8) was added. Bacterial pellet was obtained after 10 minute centrifugation. 16S rRNA gene was amplified using DNA polymerase (KOD FX). PCR Reaction mix consisted of dNTP (2 mM), both primers (50 uM) 27F (5ʹ-AGAGTTTGATCCTGGCTCAG-3ʹ) as well as 1492R (5ʹ-AGGCTACCTTGTTACGACTT-3ʹ) and 2 µl of probiotic bacterial DNA. PCR set for 35 cycles was accomplished, each comprising of denaturation (10-seconds at 98 oC), annealing (30-second at 53 oC) and extension (1-minute at 72 oC). PCR products were purified and partially sequenced employing automated sequencer.
The sequences were assayed for homology determination using BLAST-GenBank database (http://www.ncbi.nlm.nih.gov/blast) and procured for the accession numbers.
Pathogenicity of P. fluorescens in L. rohita fingerlings
L. rohita fingerlings were got from the fish farm at Muridkey, Pakistan in August. Initially, they were acclimatized to the experimental conditions for the first 10 days in Environmental Microbiology Laboratory, University of the Punjab, Lahore. Fish health status was keenly observed under experimental conditions during the experimental period.
The L. rohita fingerlings were challenged to P. fluorescens bath initially, but it resulted neither in the appearance of any disease nor mortality. Hence, intraperitoneal (i.p) injection of the pathogen was done by following the methods established by Romalde et al. (1996) and Robertson et al. (2000). For this purpose, overnight grown young culture of P. fluorescens was centrifuged and suspended subsequently in PBS to obtain O.D. of 1.0, 0.5, 0.25, 0.1, 0.05 spectrophotometrically that were subjected to viable counting for evaluating the C.F.U.ml-1 as 61 ×109, 181 ×105, 45×105, 268×103, 170×103 respectively.
To inspect the pathogenicity of the fish pathogen P. fluorescens, ten L. rohita fingerlings in each group were injected intraperitoneally with varying doses of virulent bacterium. All groups of fishes were given i.p injections of 0.1 ml pathogen suspended in PBS representing a set dose of a pathogen. The control fish group was given i.p injection of sterile PBS without fish pathogen similarly. Every group was kept in a separate aquarium at RT (27-30 ºC) having 10 liters of fresh water and was aerated continuously employing air stone. One third of the water in each aquarium was routinely changed every twenty four h and the debris/feces were also siphoned off. All fish groups were observed up to 30 days. They were given sterile fish feed and checked for mortalities daily. Pathogen concentration with of 268×103 C.F.U.ml-1 causing 50% mortality was chosen for subsequent experiment.
Probiotic treatment of pathogen challenged fish
The probiotic bacterial isolates Sphingomonas sp. AsCh-P3 and Bacillus aerius AsCh-A7 with greater antagonistic activities for P. fluorescens in in vitro studies were further scrutinized for the in vivo assays. The experiments were performed by following the methods established by Gram et al. (1999) and Spanggaard et al. (2001).
For in vivo assay, the negative (C1) and positive control (C2) groups were fed with control feed 15 days and 30 days after i.p pathogenic injections. In the experimental groups G1a, G2a control feed was administered for 15 days prior pathogenic dose followed by Sphingomonas sp. AsCh-P3 as well as Bacillus aerius AsCh-A7 treated feed for 30 days, whereas G1c, G2c was provided with the respective probiotic supplemented feed prior (15 days) and control feed after (30 days) pathogenic dose. Experimental groups G1b, G2b received Sphinogomonas sp. AsCh-P3 and Bacillus aerius AsCh-A7 enriched feed prior (15 days) and after (30 days) pathogenic dose.
All the control as well as experimental fish group consisted of triplicates with 10 fishes per replicate. At the experimental start, different groups were given simple sterilized or corresponding probiotic amalgamated feed for fifteen days. Every experimental fish was given i.p injections with a pathogen suspension of 0.1 ml. The positive control fish group was given fish pathogen whereas the negative control group was subjected to i.p injection with 0.1 ml of PBS only. Mortalities in all the aquaria (at RT of 24 to 27 ºC) were routinely noted every 24 h, whereas the dead fishes were removed continuously from each aquaria. Further, dead fishes were investigated clinically for pathogen infection. Following calculations were made for evaluation of relative percent survival (RPS) as established by Amend, 1981.
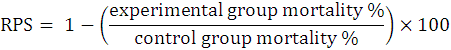
Statistical analysis
Experimental data were analyzed employing one way analysis of variance (ANOVA) following Duncan’s multiple range test (SPSS ver.18.0, SPSS, Chicago, IL, USA).
RESULTS
In vitro antagonistic potentialof antibiotic bacterial isolates against pathogen P. fluorescens
Initially 20 bacteria were scrutinized for their antibiotic potential against fish pathogen P. fluorescens using cross streak method (Table I). As shown in Table I, bacterial isolates exhibited antagonism against the fish pathogen while remaining 10 isolates could not express any growth antagonism to the fish pathogen.
Based on the GIZ by cell-free broth of the positive probiotic isolates, six bacterial isolates were selected for further study. The best inhibition zone was 15 mm, 12 mm by Sphingomonas sp. AsCh-P3 and 17 mm, 13 mm by Bacillus aerius AsCh-A7 against P. fluorescens as recorded by disc and well diffusion methods respectively. Whereas the remaining selected bacterial isolates showed inhibition zones (10-12 mm) as shown in Table II.
Growth antagonism of the selected probiotic bacteria against varying doses of fish pathogen
Six probiotic bacterial isolates of known enumeration (C.F.U.ml-1) were streaked perpendicularly to the inocula of different concentrations of fish pathogen (Table III). Antibiosis was shown by Sphingomonas sp. AsCh-P3 and Bacillus aerius AsCh-A7 against P. fluorescens at all strengths. A general antibiosis trend reflected reasonable growth inhibition for the fish pathogens cross streaked with inocula containing a less number of C.F.U.ml-1.
Table I. Antagonistic activity of different probiotic isolates against fish pathogen Pseudomonas fluorescens by cross streak method.
|
Probiotic isolates |
P. fluorescens |
|
P. mallei AsCh-P1 |
− |
|
Sphingomonas sp.AsCh-P3 |
++ |
|
P. pseudomallei AsCh-P4 |
− |
|
Enterobacter sakazaki AsCh-P6 |
− |
|
Edwardsiella hoshinae AsCh-P8 |
− |
|
P. pseudomallei AsCh-P9 |
− |
|
P. gladioli AsCh-P10 |
+ |
|
P. putida AsCh-P13 |
− |
|
P. pseudomallei AsCh-P14 |
+ |
|
P. aeruginosa AsCh-P15 |
− |
|
Sporolactobacillus inilunis AsCh-L6 |
+ |
|
Aeromonas caviae AsCh-L14 |
− |
|
Bacillus cereus AsCh-A2 |
− |
|
Listeria murnyi AsCh-A3 |
± |
|
P. pseudoalcaligenes AsCh-A4 |
+ |
|
Kurthia gibsonii AsCh-A5 |
+ |
|
Enterobacter agglomerans AsCh-A6 |
± |
|
Bacillus aerius AsCh-A7 |
++ |
|
Enterobacter aerogenes AsCh-A8 |
− |
|
Bacillus cereus AsCh-A9 |
+ |
+, Pathogens inhibited by probiotic isolates; ++, Pathogens inhibited strongly by probiotic isolates; ±, Pathogens inhibited weakly by probiotic isolates; −, Pathogens not inhibited by probiotic isolates.
Table II. Growth inhibition of different probiotic bacteria by cell free culture against the fish pathogen Pseudomonas fluorescens.
|
Probiotic Isolates |
Disca diffusion method |
Wellb diffusion method |
|
Sphingomonas sp. AsCh-P3 |
15 |
12 |
|
P. gladioli AsCh-P10 |
10 |
10 |
|
P. pseudomallei AsCh-P14 |
11 |
11 |
|
Listeria murnyi AsCh-A3 |
11 |
10 |
|
P. pseudoalcaligenes AsCh-A4 |
10 |
10 |
|
Kurthia gibsonii AsCh-A5 |
11 |
10 |
|
Enterobacter agglomerans AsCh-A6 |
10 |
10 |
|
Bacillus aerius AsCh-A7 |
17 |
13 |
|
Bacillus cereus AsCh-A9 |
11 |
10 |
|
Sporolactobacillus inilunis AsCh-L6 |
10 |
10 |
Values represent diameter of growth inhibition zones in mm; Isolates were selected based on antagonism against both pathogens and were optimized for further parameters. a, diameter of each disc required = 9mm; b, diameter of each well = 9mm.
The viable counting of the probiotic isolates in formulated fish feed
Intervening period involved in storage and transportation, etc. of feed enriched with probiotics is regarded very critical for effectual administration to an animal. This study was conducted to evaluate the viability of the bacterial strains in formulated fish feed, followed by drying at room temperature (3 days) and storage at 4 oC (4 days) (Table IV).
The selected probiotic isolates Sphingomonas sp. AsCh-P3 and Bacillus aerius AsCh-A7 showed the maximum survival rate up to 63.5 and 70.17% in fish feed respectively (Table V). Both bacterial isolates exhibited the highest growth with 30% inocula at RT (Fig. 1).
Identification and characterization of select probiotic isolates
Conspicuous colonial growth of bacterial isolate AsCh-P3 on a solidified nutrient plate showed an irregular round, raised, creamy white with wavy margins, 2.5 mm in diameter, opaque and butyreus in consistency while colonies of probiotic isolate AsCh-A7 on solidified nutrient medium were round, convex, yellowish off white with entire margins, 1-1.5 mm in diameter, transparent and semi viscous in consistency.
Bacterial isolate AsCh-P3 (G-negative) and AsCh-A7 (G-positive) were facultative anaerobe, rod shape with rounded ends with size of 1.5×1 µm and 2×1 µm containing 2 and tuft of polar flagella respectively. Both isolates did not necessitate NaCl to grow, but tolerated salt concentration (up to 6%). The bacteria were found to grow at 25, 37 and 45 °C, reduced nitrate salt with no gas emission, positive catalase, oxidase and Voges Proskauer tests. Both strains showed good growth at MacConkey agar medium but cannot grow on cetrimide agar. Test for urease is negative for AsCh-P3 and positive for AsCh-A7. AsCh-P3 showed positive methyl red test while negative by AsCh-A7. AsCh-P3 cannot grow, but AsCh-A7 showed good growth at Simmon’s citrate agar medium.
Table III. Bactericidal activity of selected probiotic isolates against different inocula of Pseudomonas fluorescens by cross streak method.
|
Probiotic isolates |
Inoculum C.F.U.ml-1 |
Different concentrations of P. fluorescens |
||||
|
61×109 (1.0) |
181×105 (0.5) |
45×105 (0.25) |
268×103 (0.1) |
170×103 (0.05) |
||
|
Sphingomonas sp. AsCh-P3 |
193×105 (0.5) |
± |
+ |
+ |
+ |
+ |
|
Pseudomonas pseudomallei AsCh-P14 |
282×105 (0.5) |
± |
± |
+ |
+ |
+ |
|
Listeria murnyi AsCh-A3 |
217×105 (0.5) |
− |
± |
± |
± |
± |
|
Kurthia gibsonii AsCh-A5 |
174×105 (0.5) |
− |
± |
± |
± |
± |
|
Bacillus aerius AsCh-A7 |
185×105 (0.5) |
± |
+ |
+ |
+ |
++ |
|
Bacillus cereus AsCh-A9 |
207×105 (0.5) |
− |
− |
± |
± |
± |
Figures in parenthesis showed the bacterial concentrations suspended in phosphate buffer saline at 600 nm. +, Pathogens inhibited by probiotic isolates; ±, Pathogens inhibited weakly by probiotic isolates; −, Pathogens not inhibited by probiotic isolates.
Table IV. Viable counting (C.F.U.g-1) of probiotic supplemented fish feed (suspension at 0.5 O.D. 600 nm) followed by drying up to 3 days and 4 days refrigerator storage.
|
Probiotic isolates |
Days |
||||
|
0a |
1 |
2 |
3 |
7 |
|
|
Sphingomonas sp. AsCh-P3 |
93×106 |
38×106 |
125×104 |
63×104 |
40×104 |
|
P. pseudomallei AsCh-P14 |
212×106 |
102×106 |
256×104 |
112×104 |
70×104 |
|
Listeria murnyi AsCh-A3 |
177×106 |
53×106 |
85×104 |
42×104 |
23×104 |
|
Kurthia gibsonii AsCh-A5 |
114×106 |
36×106 |
70×104 |
33×104 |
20×104 |
|
Bacillus aerius AsCh-A7 |
150×106 |
50×106 |
132×104 |
57×104 |
40×104 |
|
Bacillus cereus AsCh-A9 |
167×106 |
66×106 |
249×104 |
134×104 |
43×104 |
a, 20% (v/w) of each bacterial suspension was mixed in sterilized fish feed.
Table V. Percent survival rate of probiotics in bacterial enriched, dried and stored fish feed.
|
Probiotic isolates |
Days |
||||
|
0* |
1* |
2a |
3a |
7a |
|
|
Sphingomonas sp. AsCh-P3 |
100 |
40.86 |
3.29 |
50.4 |
63.5 |
|
P. pseudomallei AsCh-P14 |
100 |
48.11 |
2.5 |
43.75 |
62.5 |
|
Listeria murnyi AsCh-A3 |
100 |
29.94 |
16.03 |
49.41 |
54.76 |
|
Kurthia gibsonii AsCh-A5 |
100 |
31.58 |
1.94 |
47.14 |
60.6 |
|
Bacillus aerius AsCh-A7 |
100 |
39.52 |
3.77 |
43.18 |
70.17 |
|
Bacillus cereus AsCh-A9 |
100 |
33.33 |
2.64 |
53.81 |
32.09 |
* % survival rate was calculated as; (CFU/g in feed after drying/ CFU/g in feed before drying at previous day)×100
Table VI. Mortality of L. rohita fingerlings challenged with intraperitoneal P. fluorescens pathogen within one month.
|
Groups |
Bacterial Conc (O.D) |
C.F.U. ml-1 |
Days post inoculations |
|||||||
|
1 |
2 |
3 |
4c |
5c |
10c |
20c |
30c |
|||
|
Control |
PBSa |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
|
|
P. fluorescens |
1.00 |
61×109 b |
10/10 |
|||||||
|
0.5 |
181×105 |
10/10 |
||||||||
|
0.25 |
45×105 |
8/10 |
2/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
|
|
0.1 |
268×103 |
2/10 |
2/10 |
1/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
|
|
0.05 |
170×103 |
2/10 |
1/10 |
1/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
|
a, Control were injected with 0.1 ml of sterile PBS /fish; b, Experimental groups were injected with 0.1 ml of respective dose / fish; c, No mortality was recorded in control and experimental groups.; PBS, Phosphate buffer saline.
Morphological identification, biochemical tests as well as the molecular characterization involving 16S rRNA gene sequencing were performed on both probiotic isolates. It was found that isolate AsCh-P3 and AsCh-A7 exhibited 99% and 98% similarity index to 16S rRNA gene sequence of Sphingomonas sp. and Bacillus aerius respectively. The accession numbers MF543123 and MF543123 were assigned to the isolates Sphingomonas sp. AsCh-P3 and Bacillus aerius AsCh-A7, respectively.
Pathogenecity of P. fluorescens and effect of probiotics
Mortalities of Labeo rohita fingerlings injected with P. fluorescens in 30 day experiment showed a dose dependent manner (Table VI). Pathogen concentration that consequent in 50% mortality was scrutinized for subsequent study. The fish groups exposed to P. fluorescens manifested symptoms like swollen body cavity, protrusion and reddening of the anus, damaged scales, erythma of eyes and inflated belly as presented in Figure 2.
The P. fluorescens injected fingerlings were given control and probiotics amalgamated feeds in multiple experiments and animals were observed carefully for mortalities (up to 30 days) as recorded in Table VII. It appeared that provision of probiotics in feed before and after the pathogen injection (G1b, G2b) caused a significant decrease in the mortalities (20 and 10 %) as compared to respective positive control (C2). Similarly, the mortalities reduced significantly, i.e., 26.67 and 13.34% for having a probiotic feed prior pathogen exposure (G1c, G2c). But 46.67 and 26.67% mortalities were recorded in the groups receiving the probiotics containing feeds soon after the pathogen administration (G1a, G2a). Highest RPS of 60 and 80% appeared in G1b and G2b fishes fed with Sphingomonas sp. AsCh-P3 and Bacillus aerius AsCh-A7 augmented feed before and after the pathogen injection. Probioticity of the isolates was exhibited by improved RPS in the experimental fish groups (Table VIII).
Table VII. Mortality of L. rohita fingerlings challenged with intraperitoneal P. fluorescens pathogen within one month and fed with probiotic augmented feed under different experimental conditions.
|
Groups |
Group code |
Days post challenge |
|||||||
|
1 |
2 |
3 |
4 |
5 |
10 |
20 |
30 |
||
|
Negative control-PBS |
(C1) |
a0±0.00 |
a0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
|
Positive control- P.fluorescens |
(C2) |
b20.00 |
b2±0.58 |
1±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
|
P.fluorescens+ Sphingomonas sp. AsCh-P3 |
(G1a) |
b2±0.00 |
Ab1.66±0.33 |
1±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
|
Sphingomonas sp. AsCh-P3 + P.fluorescens+ Sphingomonas sp. AsCh-P3 |
(G1b) |
a0.66±0.33 |
Ab0.66±0.33 |
0.66±0.33 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
|
Sphingomonas sp.AsCh-P3 +P.fluorescens +simple feed |
(G1c) |
c1±0.00 |
Ab1±0.58 |
1±0.58 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
|
P.fluorescens + B. aerius AsCh-A7 |
(G2a) |
c1±0.00 |
Ab1±0.00 |
ab0.66 ±0.33 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
|
B. aerius AsCh-A7+P. fluorescens + B. s aerius AsCh-A7 |
(G2b) |
ac0.33±0.33 |
Ab0.66 ±0.33 |
a0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
|
B. aerius AsCh-A7+P. fluorescens + simple feed |
(G2c) |
ac0.66±0.33 |
Ab0.66 ±0.33 |
a±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
0±0.00 |
All values represent means of three replicates ±S.E.M. Two values within a column not sharing a common alphabet differ significantly from both control and same experimental groups. Values are significantly different at p≤ 0.5 at single factor analysis of variance.
Table VIII. Relative Percent Survival in P. fluorescens challenged L. rohita fingerlings intraperitoneally within one month and fed with probiotics augmented feed.
|
Groups |
Group code |
% Mortality rate |
% Survival rate |
RPS (%) |
|
Negative control-PBS |
(C1) |
a0±0.00 |
a100±0.00 |
|
|
Positive control- P. fluorescens |
(C2) |
b50±5.78 |
b50±5.78 |
|
|
P. fluorescens+ Sphingomonas sp. AsCh-P3 |
(G1a) |
b46.66±3.33 |
b53.33±3.33 |
6.66 |
|
Sphingomonas sp. AsCh-P3 +P. fluorescens+ Sphingomonas sp. AsCh-P3 |
(G1b) |
c20±0.00 |
c80±0.00 |
60.00 |
|
Sphingomonas sp. AsCh-P3 +P. fluorescens +simple feed |
(G1c) |
c26.66±3.33 |
c73.33±3.33 |
46.66 |
|
P. fluorescens + B. aerius AsCh-A7 |
(G2a) |
c26.66±3.33 |
c73.33±3.33 |
46.66 |
|
B. aerius AsCh-A7+P. fluorescens + B. aerius AsCh-A7 |
(G2b) |
a10±0.00 |
a90±0.00 |
80.00 |
|
B. aerius AsCh-A7+P.f luorescens +simple feed |
(G2c) |
ac13.33±3.33 |
ac86.66±3.33 |
73.32 |
All values represent means of three replicates ±S.E.M. Two values within a column not sharing a common alphabet differ significantly from both control and same experimental groups. Values are significantly different at p≤ 0.5 at single factor analysis of variance. RPS, Relative percent survival.
Asymptomatic surviving fish of negative and positive control as well as experimental groups challenged with the pathogen and treated with Sphingomonas sp. AsCh-P3 and Bacillus aerius AsCh-A7 were shown in Figure 3, respectively. Caudal fin deformation was observed in G2a group in which Bacillus subtilis AsCh-A7 supplemented feed was administered after pathogen challenge while muscle degeneration was recorded in group G1b and G2b provided with Sphingomonas sp. AsCh-P3 and Bacillus aerius AsCh-A7 treated feed before and after pathogen challenge (Fig. 4).
DISCUSSION
The present study investigated the bactericidal and bacteriostatic potential of probiotic isolates against Pseudomonas fluorescens (a pathogen of Labeo rohita) following cross streak, disc and well diffusion method. Production of inhibitory compounds in in vitro assay is helpful in the scrutiny of probiotic microbe (Gram et al., 1999). The possible mode of action of probiotics to exert antibacterial phenomenon may be due to antimicrobial peptide, bacteriocin, siderophore, lysozyme, proteases and production of organic acids to lower pH (Van Hai et al., 2009; Heo et al., 2013; Ferreira et al., 2015). The bactericidal activity of Bacillus licheniformis and B. pumilus was affected by high bile concentration and low pH (Ramesh et al., 2015). Pathogen growth and cell density was reduced by extracellular products (ECPs) which is the main virulent factor. Lactobacillus plantarum 44a and Lactobacillus lactis 18f inhibited Aeromonas hydrophila, E. tarda and Staphylococcus aureus with inhibition zones 7-12 mm (Rengpipat et al., 1998).
P. fluorescens showed host specificity when the bacteria were given i-p injections in L. rohita fingerlings. Fishes were found susceptible to Pseudomonas septicemia. The majority of the dead fingerlings exhibited hemorrhages in the intestine, anal as well as body cavity inflammation and reddening of ventral body surface. In Pseudomonas septicemia, the observed symptoms were swollen body cavity, reddening of anus, belly inflated and erythma of eyes. It was inferred that the fish mortalities were concordant to the concentration of the fish pathogen in the intraperitoneal injections.
Regarding the bath exposure experiments performed with rohu fingerlings, no mortality was recorded even in groups with abraded skin for pathogen after 30 days post inoculation. Interestingly, the inoculated abraded fish for pathogen become normal within 30 days experimentation period. In a comparable study, Romalde et al. (1996) and Rasheed and Plumb (1984) were unable in reproducing the infection even in injured Gulf Killifish bathed in probiotic bacterial culture. Although here are numerous hypotheses related to the entry routes of pathogen in fish; as described in live feed by pathogens orally in turbot post larvae (Grisez et al., 1997). Also, skin, gills and anus were considered important entry portals of pathogen into eel (Chart and Munn, 1980), rainbow trout (Laurencin and Germon, 1987) and ayu (Kanno et al., 1989). Generally, failures of occurrence of disease in bathing experiments demonstrate the strong defensive mechanism of healthy fishes. Intraperitoneal inoculation of single cell protein of B. pumilus and B. licheniformis boosted the immunity against Aeromonas hydrophila infected L. rohita (Ramesh et al., 2015). However, bacterial pathogenic infections are most likely to happen in aquaculture. Interest in the use of fish probiotics has received increasing attention during the last decades.
Though fish mortalities in experimental groups that were given probiotics supplemented diet only after bacterial pathogen administration were higher than the fish group that was fed with probiotic enriched feed both prior and after pathogen administration but significantly lesser mortalities of the former group compared with the positive control group. This elucidated that probiotics might be competitive in colonizing their host, but they require more number of probiotic cells for enhancing their antipathogenic efficiencies. It is regarded that the presumed colonization of probiotics may have inhibited the growth and consequent virulence of the pathogen. Probiotics were proved as promising candidates in treating host GIT inflammation and preventing diseases (Azimirad et al., 2016; Modanloo et al., 2017). The presumed mechanisms may be nutrient competition (Ringo et al., 2016), adhesion to mucosal epithelium of GIT (Luis-Villaseñor et al., 2011), competitive exclusion of probiotics with intestinal epithelium and mucus to prevent pathogen colonization (Mahdhi et al., 2012; Sorroza et al., 2012) elevated feed digestibility by improving digestive enzymes (Zokaeifar et al., 2012), production of bacteriocin, fatty acids, organic acids and vitamin B12 (Vine et al., 2006).
Probiotics compete for adhesion receptor to reduce colonization of pathogen by antagonistic activity (Chabrillón et al., 2005; Luis-Villaseñor et al., 2011), hence proved to be as substitute for chemotherapeutants and antibiotics (Cheng et al., 2014). Probiotics have antagonistic activity by exerting competition of nutrients against pathogens (Ringo et al., 2016). Innate immunity was also be improved by probiotics by stimulating serum peroxidase, lysozyme and blood respiratory burst activities by lactobacillus lactis against Streptococcus iniae and Pseudomonas fluorescens in Nile tilapia and olive flounder (Heo et al., 2013; Merrifield and Carnevali, 2014; Beck et al., 2015).
Dietary administered B. subtilis and P. aeruginosa VSG-2 in concentrations of 1.5 × 107 and 107- 109 CFU g−1 improve immune response against A. hydrophyla in L. rohita (Kumar et al., 2006; Giri et al., 2012) while B. subtilis AB1 supplemented diet in different ways (viable or sonicated or cell free supernantant) per se protected rainbow trout against Aeromonas (Newaj-Fyzul et al., 2007). Bacillus genus contributes to promote growth, immune response and protect from diseases and reviewed for immunomodulation potential extensively (Mingmongkolchai and Panbangred, 2018).
Probiotics can be administered as multi strain or coculture to boost up the growth and immune response. B. subtilis L10 and G1 were resulted as immune stimulant and growth promoter in juvimile white shrimp against Vibrio harveyi when inoculated as combined culture (Zokaeifar et al., 2012). B. subtilis strain S12 inhibited V. harveyi in white shrimp, Litopenaeus vannamei in form of monoculture (Liu et al., 2014).
The literature evidenced the antagonistic potential of Bacillus sp. against aquatic pathogens as well as Acinetobacter sp. KX775221, Acinetobacter tandoii KX775222 and Aeromonas hydrophila KX756709 (Kaynar and Beyatli, 2012; Ramesh et al., 2015). B. amyloliquefaciens showed antagonism to control vibriosis in turbot Scophthalmus maxima (Chen et al., 2016a, b), Nile tilapia Oreochromis niloticus (Selim and Reda, 2015), catfish Ictalurus punctatus (Ran et al., 2012), European eel Anguilla anguilla (Cao et al., 2011) and Catla catla (Das et al., 2013).
CONCLUSION
The study was an attempt to evaluate the inhibitory effects of probiotic bacterial isolates Sphingomonas sp. AsCh-P3 and Bacillus aerius AsCh-A7 against fish pathogen Pseudomonas fluorescens. The outcomes revealed that the use of the probiotics augmented fish feed as demonstrated in this study will be highly beneficial for ensuring the fish health in future.
ACKNOWLEDGEMENT
This research work is a part of the first author’s Ph.D work and is grateful to University of the Punjab for financial support.
Ethical approval
It is to certify that during experimental research, all applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Statement of conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES
Abraham, T.J. and Banerjee, T., 2007. Beneficial antagonistic bacteria from freshwater fishes and culture environment as probiotics in ornamental fish culture. Indian J. Fish., 54: 311-9.
Amend, D.F., 1981. Potency testing of fish vaccines. International Symposium of Fish Biologics: Sero diagnostics and Vaccines. Dev. Biol. Stand., 49: 447-454.
Arıg, N., Suzer, C., Gokvardar, A., Basaran, F., Coban, D., Yıldırım, S., Kamaci, H.O., Fırat, K and Saka, S., 2013. Effects of probiotic (Bacillus sp.) supplementation during larval development of Gilthead sea bream (Sparus aurata, L.). Turk. J. Fish aquat. Sci., 13: 407-414. https://doi.org/10.4194/1303-2712-v13_3_03
Austin, B., Baudet, E. and Stobie, M., 1992. Inhibition of bacterial fish pathogens by Tetraselmis suecica. J. Fish Dis., 15: 55-61. https://doi.org/10.1111/j.1365-2761.1992.tb00636.x
Authira, R.R., Kirithika, M., Venkatesan, S. and Ganesan, R., 2011. Study on the in vivo and in vitro antagonistic activity of probiotics against fish pathogens. J. biol. Res., 4: 271-275.
Azimirad, M., Meshkini, S., Ahmadifard, N. and Hoseinifar, S.H., 2016. The effects of feeding with synbiotic (Pediococcus acidilactici and fructooligosaccharide) enriched adult Artemia on skin mucus immune responses, stress resistance, intestinal microbiota and performance of angelfish (Pterophyllum scalare). Fish Shellf. Immun., 54: 516–522. https://doi.org/10.1016/j.fsi.2016.05.001
Beck, B.R., Kim, D., Jeon, J., Lee, S.M., Kim, H.K., Kim, O.J., Lee, J.I., Suh, B.S., Do, H.K., Lee, K.H., Holzapfel, W.H., Hwang, J.Y., Kwon, M.G. and Song, S.K., 2015. The effects of combined dietary probiotics Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 on innate immunity and disease resistance in olive flounder (Paralichthys olivaceus). Fish Shellf.Immunol., 42: 177–183. https://doi.org/10.1016/j.fsi.2014.10.035
Benson, H.J., 2002. Microbiological applications: A laboratory manual in general microbiology. 8th edn. McGraw Hill, New York.
Cao, H., He, S., Wei, R., Diong, M. and Lu, L., 2011. Bacillus amyloliquefaciens G1: A potential antagonistic bacterium against eel-pathogenic Aeromonas hydrophila. Evid. Based Complement. Altern. Med., 2011: 824104. https://doi.org/10.1155/2011/824104
Chabrillón, M., Rico, R., Arijo, S., Díaz-Rosales, P., Balebona, M., and Moriñigo, M., 2005. Interactions of microorganisms isolated from gilthead sea bream, Sparus aurata L., on Vibrio harveyi, a pathogen of farmed Senegalese sole, Solea senegalensis (Kaup). J. Fish. Dis., 28: 531–537. https://doi.org/10.1111/j.1365-2761.2005.00657.x
Chart, H. and Munn, C.B., 1980. Experimental vibriosis in the eel (Anguilla anguilla). In: Fish diseases. Proc. Life Sci. Springer, Heidelberg, Berlin. pp. 39-44. https://doi.org/10.1007/978-3-642-67854-7_7
Chaudhary, A. and Qazi J.I., 2011. Prevalence of Pseudomonas and Lactic acid bacteria in raw milk and yogurt sampled from Lahore. Proc. Pak. Congr. Zool., 31: 79-87.
Chen, Y., Li, J., Xiao, P., Li, G.Y., Yue, S., Huang, J., Zhu, W.Y. and Mo, Z.L., 2016a. Isolation and characterization of Bacillus spp. M001 for potential application in turbot (Scophthalmus maximus L) against Vibrio anguillarum. Aquacult. Nutr., 22: 374–381. https://doi.org/10.1111/anu.12259
Chen, Y., Li, J., Xiao, P., Zhu, W. and Mo, Z.L., 2016b. The ability of marine Bacillus spp. isolated from fish gastrointestinal tract and culture pond sediment to inhibit growth of aquatic pathogenic bacteria. Iran. J. Fish Sci., 15: 701–714.
Cheng, G., Hao, H., Xie, S., Wang, X., Dai, M., Huang, L. and Yuan, Z., 2014. Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Front. Microbiol., 5: 217. https://doi.org/10.3389/fmicb.2014.00217
Collins, C.H., Lyne, P.M. and Grange, J.M., 1995. Collins and Lyne’s microbiological methods. Butterworth–Heinemann, Oxford.
Dahiya, T., Gahlawat, S.K. and Sihag, R.C., 2012. Elimination of pathogenic bacterium (Micrococcus sp.) by the use of probiotics. Turk. J. Fish aquat. Sci., 12: 185-187. http://www.trjfas.org/uploads/pdf_559.pdf, https://doi.org/10.4194/1303-2712-v12_1_21
Dahiya, T.P., Kant, R. and Sihag, R.C., 2010. Use of probiotics as an alternative method of disease control in aquaculture. Biosphere, 2: 52-57.
Das, A., Nakhro, K., Chowdhury, S. and Kamilya, D., 2013. Effects of potential probiotic Bacillus amyloliquifaciens FPTB16 on systemic and cutaneous mucosal immune responses and disease resistance of catla (Catla catla). Fish Shellf. Immunol., 35: 1547–1553. https://doi.org/10.1016/j.fsi.2013.08.022
Dawood, M.A.O., Koshio, S., Ishikawa,M., Yokoyama, S., El Basuini, M.F., Hossain, M.S., Nhu, T.H., Dossou, S. and Moss, A.S. 2016. Effects of dietary supplementation of Lactobacillus rhamnosus or/and Lactococcus lactis on the growth, gut microbiota and immune responses of red sea bream, Pagrus major. Fish Shellf. Immunol., 49: 275–285. https://doi.org/10.1016/j.fsi.2015.12.047
Denev, S., Staykov, Y., Moutafchieva, R. and Beev, G., 2009. Microbial ecology of the gastrointestinal tract of fish and the potential application of probiotics and prebiotics in finfish aquaculture. Int. aquat. Res., 1: 1-29.
Divya, J.B., Varsha, K.K., Nampoothiri, K.M., Ismail, B. and Pandey, A., 2012. Probiotic fermented foods for health benefits. Eng. Life Sci., 12: 377-390. https://doi.org/10.1002/elsc.201100179
Esteban, M.Á., Cordero, H., Martínez-Tomé, M., Jiménez-Monreal, A.M.; Bakhrouf, A. and Mahdhi, A., 2014. Effect of dietary supplementation of probiotics and palm fruits extracts on the antioxidant enzyme gene expression in the mucosae of gilthead seabream (Sparus aurata L.). Fish Shellf. Immunol., 39: 532–540. https://doi.org/10.1016/j.fsi.2014.06.012
Ferreira, G.S., Bolívar, N.C., Pereira, S.A., Guertler, C., do Nascimento Vieira, F., Mouriño, J.L.P. and Seiffert, W.Q., 2015. Microbial biofloc as source of probiotic bacteria for the culture of Litopenaeus vannamei. Aquaculture, 448: 273–279. https://doi.org/10.1016/j.aquaculture.2015.06.006
Ghosh, S., Sinha, A. and Sahu, C., 2007. Isolation of putative probionts from the intestines of Indian major carps. Isr. J. Aquacult. Bamidgeh, 59: 127–132.
Giri, S.S., Sen, S.S. and Sukumaran, V., 2012. Effects of dietary supplementation of potential probiotic Pseudomonas aeruginosa VSG-2 on the innate immunity and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellf. Immunol., 32: 1135–1140. https://doi.org/10.1016/j.fsi.2012.03.019
Gobi, N., Malaikozhundan, B., Sekar, V., Shanthi, S., Vaseeharan, B., Jayakumar, R. and Nazar, A.K., 2016. GFP tagged Vibrio parahaemolyticus Dahv2 infection and the protective effects of probiotic Bacillus licheniformis Dahb1 on the growth, immune and antioxidant responses in Pangasius hypophthalmus. Fish Shellf. Immunol., 52: 230–238. https://doi.org/10.1016/j.fsi.2016.03.006
Gobinath, J. and Ramanibai, R., 2012. Effect of probiotic bacteria culture on pathogenic bacteria from fresh water fish Oreochromis massambicus. J. Mod. Biotechnol., 1: 50-54.
Golic, N., Veljovic, K., Popovic, N., Djokic, J., Strahinic, I., Mrvaljevic, I. and Terzić-Vidojevic, A., 2017. In vitro and in vivo antagonistic activity of new probiotic culture against Clostridium difficile and Clostridium perfringens. BMC Microbiol., 17: 108. https://doi.org/10.1186/s12866-017-1015-5
Gomez-Gil, B., Roque, A. and Turnbull, J.F., 2000. The use and selection of probiotic bacteria for in the culture of larval aquatic organisms. Aquaculture, 191: 259-70. http://citeseerx.ist.psu.edu/viewdoc/download? https://doi.org/10.1016/S0044-8486(00)00431-2
Gram, L., Melchiorsen, J., Spanggaard, B. and Huber, I., 1999. Inhibition of Vibrio anguillarum by Pseudomonas fluorescens AH2: A possible probiotic treatment of fish. Appl. environ. Microbiol., 65: 969-73. https://www.ncbi.nlm.nih.gov/pubmed/10049849, https://doi.org/10.1128/AEM.65.3.969-973.1999
Grisez, L., Reyniers, J., Verdonck, L. and Swings, J. 1997. Dominant intestinal microflora of sea bream and sea bass larvae, from two hatcheries, during larval development. Aquaculture, 155: 387-99. https://doi.org/10.1016/S0044-8486(97)00113-0
Heo, W.S., Kim, Y.R., Kim, E.Y., Bai, S.C. and Kong, I.S., 2013. Effects of dietary probiotic, Lactococcus lactis subsp. lactis I2, supplementation on the growth and immune response of olive flounder (Paralichthys olivaceus). Aquaculture, 376: 20–24. https://doi.org/10.1016/j.aquaculture.2012.11.009
Holt, J.G., Krieg, N.R., Sneath, P.H.A., Stanley, J.T. and William, S.T., 1994. Bergey’s manual of determinative bacteriology. Williams and Wilikins, Baltimore.
Irianto, A. and Austin, B., 2002. Probiotics in aquaculture. J. Fish Dis., 25: 633-642. https://doi.org/10.1046/j.1365-2761.2002.00422.x
Ishibashi, N. and Yamazaki, S., 2001. Probiotics and safety. Am. J. Clin. Nutr., 73 465-470. https://doi.org/10.1093/ajcn/73.2.465s
Itoh, T., Fujimoto, Y., Kawai, Y. and Toba, T., 1995. Inhibition of food-borne pathogenic bacteria by bacteriocins from Lactobacillus gasseri. Lett. appl. Microbiol., 21: 137-41. https://www.ncbi.nlm.nih.gov/pubmed/7576495, https://doi.org/10.1111/j.1472-765X.1995.tb01025.x
Jones, P.J., 2002. Clinical nutrition: 7 Functional foods more than just nutrition. Can. med. Assoc. J., 166: 1555-1563.
Kanno, T., Nakai T. and Muroga, K., 1989. Mode of transmission of vibriosis among ayu Plecoglossus altivelis. J. aquat. Anim. Hlth, 1: 2-6. https://doi.org/10.1577/1548-8667(1989)001<0002:MOTOVA>2.3.CO;2
Kaynar, P. and Beyatli, Y., 2012. Antagonistic activities of Bacillus spp. strains isolated from the fishes. J. appl. Biol. Sci., 6: 77–81.
Kolndadacha, O.D., Adikwu, I.A., Okaeme, A.N. and Atiribom, R.Y. 2001. The role of probiotics in aquaculture in Nigeria-a review. Continent. J. Fish aquat. Sci., 5: 8-15. http://aquaticcommons.org/7315/
Kumar, R., Mukherjee, S.C., Pani Prasad, K. and Pal, A.K., 2006. Evaluation of Bacillus subtilis as a probiotic to Indian major carp, Labeo rohita (Ham). Aquact. Res., 37: 1215–1221. https://doi.org/10.1111/j.1365-2109.2006.01551.x
Laurencin, F.B. and Germon, E., 1987. Experimental infection of rainbow trout, Salmo gairdneri R., by dipping in suspensions of Vibrio anguillarum: Ways of bacterial penetration; influence of temperature and salinity. Aquaculture, 67: 203-205. https://eurekamag.com/pdf/001/001591831.pdf, https://doi.org/10.1016/0044-8486(87)90028-7
Linders, L.J., Meerdink, G. and Van’t Riet, K., 1996. Influence of temperature and drying rate on the dehydration inactivation of Lactobacillus plantarum. Fd. Bioprod. Process, 74: 110-4. http://library.wur.nl/WebQuery/wurpubs/35749
Liu, H., Li, Z., Tan, B., Lao, Y., Duan, Z., Sun, W. and Dong, X., 2014. Isolation of a putative probiotic strain S12 and its effect on growth performance, non-specific immunity and disease-resistance of white shrimp, Litopenaeus vannamei. Fish Shellf. Immunol., 41: 300–307. https://doi.org/10.1016/j.fsi.2014.08.028
Luis-Villaseñor, I.E., Macías-Rodríguez, M.E., Gómez-Gil, B., Ascencio-Valle, F., and Campa-Córdova, Á.I., 2011. Beneficial effects of four Bacillus strains on the larval cultivation of Litopenaeus vannamei. Aquaculture, 321: 136–144. https://doi.org/10.1016/j.aquaculture.2011.08.036
Lom, J. and Schubert, G., 1983. Ultrastructural study of Pisdnoodinium pillulare (Schäperclaus, 1954) Lom, 1981 with special emphasis on its attachment to the fish host. J. Fish Dis., 6: 411-428.
Mahdhi, A., Kamoun, F., Messina, C., Santulli, A. and Bakhrouf, A., 2012. Probiotic properties of Brevibacillus brevis and its influence on sea bass (Dicentrarchus labrax) larval rearing. Afr. J. microbiol. Res., 6: 6487–6495. https://doi.org/10.5897/AJMR12.1201
Merrifield, D.L., and Carnevali, O., 2014. Probiotic Modulation of the Gut Microbiota of Fish. In: Aquaculture Nutrition: Gut health, probiotics and prebiotics (eds. D. Merrifield and E. Ringø). John Wiley and Sons Ltd., Hoboken, NJ. https://doi.org/10.1002/9781118897263
Mingmongkolchai, S. and Panbangred, W., 2018. Bacillus probiotics: an alternative to antibiotics for livestock production. J. appl. Microbiol., 124: 1334−1346. https://doi.org/10.1111/jam.13690
Miranda, C.D. and Rojas, R., 2007. Occurrence of florfenicol resistance in bacteria associated with two Chilean salmon farms with different history of antibacterial usage. Aquaculture, 266: 39-46. https://doi.org/10.1016/j.aquaculture.2007.02.007
Modanloo, M., Soltanian, S., Akhlaghi, M. and Hoseinifar, 2017. The effects of single or combined administration of galactooligosaccharide and Pediococcus acidilactici on cutaneous mucus immune parameters, humoral immune responses and immune related genes expression in common carp (Cyprinus carpio) fingerlings. Fish Shellf. Immunol., 70: 391–397. https://doi.org/10.1016/j.fsi.2017.09.032
Mohideen, M.M., Kader, A., Mohan, T.S. and Mohamed, S.P., 2010. Effect of probiotic bacteria on the growth rate of freshwater fish, Catla catla. Int. J. Biol. Technol., 1: 113-117. https://www.researchgate.net/publication/285698677
Muñoz-Atienza, E., Araújo, C., Magadán, S., Hernández, P.E., Herranz, C., Santos, Y. and Cintas, L.M., 2014. In vitro and in vivo evaluation of lactic acid bacteria of aquatic origin as probiotics for turbot (Scophthalmus maximus L.) farming. Fish Shellf. Immunol., 41: 570–580. https://doi.org/10.1016/j.fsi.2014.10.007
Nayak, S.K., 2010. Probiotics and immunity: A fish perspective. Fish Shellf. Immunol., 29: 2-14. https://doi.org/10.1016/j.fsi.2010.02.017
Newaj-Fyzul, A., Adesiyun, A.A., Mutani, A., Ramsubhag, A., Brunt, J. and Austin, B., 2007. Bacillus subtilis AB1 controls Aeromonas infection in rainbow trout (Oncorhynchus mykiss, Walbaum). J. appl. Microbiol., 103: 1699–1706. https://doi.org/10.1111/j.1365-2672.2007.03402.x
Nikoskelainen, S., Ouwehand, A., Salminen, S. and Bylund, G., 2001. Protection of rainbow trout (Oncorhynchus mykiss) from furunculosis by Lactobacillus rhamnosus. Aquaculture, 198: 229-236. https://doi.org/10.1016/S0044-8486(01)00593-2
Olsson, J.C., Westerdahl, A.L., Conway, P.L. and Kjelleberg, S.T., 1992. Intestinal colonization potential of turbot (Scophthalmus maximus)-and dab (Limanda limanda)-associated bacteria with inhibitory effects against Vibrio anguillarum. Appl. environ. Microbiol., 58: 55155-55156. https://doi.org/10.1128/AEM.58.2.551-556.1992
Panigrahi, A., Kiron, V., Kobayashi, T., Puangkaew, J., Satoh, S. and Sugita, H., 2004. Immune responses in rainbow trout Oncorhynchus mykiss induced by a potential probiotic bacteria Lactobacillus rhamnosus JCM 1136. Vet. Immunol. Immunopathol., 102: 379-388. https://doi.org/10.1016/j.vetimm.2004.08.006
Ramesh, D., Vinothkanna, A., Rai, A.K. and Subramanian, V., 2015. Isolation of potential probiotic Bacillus spp. and assessment of their subcellular components to induce immune responses in Labeo rohita against Aeromonas hydrophila. Fish Shellf. Immunol., 45: 268–276. https://doi.org/10.1016/j.fsi.2015.04.018
Ran, C., Carrias, A., Williams, M.A., Capps, N., Dan, B.C.T., Newton, J.C., Kloepper, J.W., Ooi, E.L., Browdy, C.L., Terhune, J.S. and Liles, M.R., 2012. Identification of Bacillus strains for biological control of catfish pathogens. PLoS One, 7: e45793. https://doi.org/10.1371/journal.pone.0045793
Rasheed, V. and Plumb, J.A., 1984. Pathogenicity of a non-haemolytic group B Streptococcus sp. in gulf killifish (Fundulus grandis Baird and Girard). Aquaculture, 37: 97-105. https://doi.org/10.1016/0044-8486(84)90067-X
Rengpipat, S., Rukpratanporn, S., Piyatiratitivorakul, S. and Menasveta, P., 1998. Probiotics in aquaculture: a case study of probiotics for larvae of the black tiger shrimp (Penaeus monodon). Adv. Shrimp Biotechnol., 177-181. http://www.nani.com.vn/upload/files/3589695250074010
Rengpipat, S., Rukpratanporn, S., Piyatiratitivorakul, S., and Menasaveta, P., 2000. Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture, 191: 271–288. https://doi.org/10.1016/S0044-8486(00)00440-3
Ringø, E., Zhou, Z., Vecino, J.L.G., Wadsworth, S., Romero, J., Krogdahl, Å., Olsen, R.E., Dimitroglou, A., Foey, A., Davies, S., Owen, M., Lauzon, H.L., Martinsen, L.L., De Schryver, P., Bossier, P., Sperstad, S. and Merrifield, D.L., 2016. Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquacult. Nutr., 22: 219–282. https://doi.org/10.1111/anu.12346
Robertson, P.A., O’Dowd, C., Burrells, C. and Williams, P., 2000. Use of Carnobacterium sp. as a probiotic for Atlantic salmon (Salmo salar L.) and rainbow trout (Oncorhynchus mykiss, Walbaum). Aquaculture, 185: 235-243. https://doi.org/10.1016/S0044-8486(99)00349-X
Romalde, J.L., Magarinos, B., Nunez, S. and Barja, J.L., 1996. Host range susceptibility of Enterococcus sp. strains isolated from diseased turbot: Possible routes of infection. Appl. environ. Microbiol., 62: 607-611. https://www.ncbi.nlm.nih.gov/pubmed/8593061, https://doi.org/10.1128/AEM.62.2.607-611.1996
Sahoo, T.K., Jena, P.K., Nagar, N., Patel, A.K. and Seshadri, S., 2015. In vitro evaluation of probiotic properties of lactic acid bacteria from the gut of Labeo rohita and Catla catla. Probiotics Antimicrob. Proteins, 7: 126–136. https://doi.org/10.1007/s12602-015-9184-8
Seenivasan, C., Bhavan, P.S., Radhakrishnan, S. and Muralisankar, T., 2012. Effects of probiotics on survival, growth and biochemical constituents of freshwater prawn Macrobrachium rosenbergii post larvae. Turk. J. Fish aquat. Sci., 12: 331-338.
Selim, K.M. and Reda, R.M., 2015. Improvement of immunity and disease resistance in the Nile tilapia, Oreochromis niloticus, by dietary supplementation with Bacillus amyloliquefaciens. Fish Shellf. Immunol., 44: 496–503. https://doi.org/10.1016/j.fsi.2015.03.004
Sihag, R.C. and Sharma, P., 2012. Probiotics: the new ecofriendly alternative measures of disease control for sustainable aquaculture. J. Fish. aquat. Sci., 7: 72. https://scialert.net/abstract/?doi=jfas.2012.72.103
Sorroza, L., Padilla, D., Acosta, F., Román, L., Grasso, V., Vega, J. and Real, F., 2012. Characterization of the probiotic strain Vagococcus fluvialis in the protection of European sea bass (Dicentrarchus labrax) against vibriosis by Vibrio anguillarum. Vet. Microbiol., 155: 369–373. https://doi.org/10.1016/j.vetmic.2011.09.013
Spanggaard, B., Huber, I., Nielsen, J. and Sick, E.B., 2001. The probiotic potential against vibriosis of the indigenous microflora of rainbow trout. Environ. Microbiol., 3: 755-65. https://www.ncbi.nlm.nih.gov/pubmed/11846769, https://doi.org/10.1046/j.1462-2920.2001.00240.x
Tabak, S., Maghnia, D. and Bensoltane, A., 2012. The Antagonistic activity of the lactic acid bacteria (Streptococcus thermophilus, Bifidobacterium bifidum and Lactobacillus bulgaricus) against Helicobacter pylori responsible for the gastroduodenals diseases. J. agric. Sci. Technol. A., 2: 709.
Uğur, A., Ceyla, O. and Aslım, B., 2012. Characterization of Pseudomonas spp. from Seawater of the Southwest Coast of Turkey. J. Biol. environ. Sci., 6: 15-23.
Van Hai, N., Buller, N. and Fotedar, R., 2009. The use of customised probiotics in the cultivation of western king prawns (Penaeus latisulcatus Kishinouye, 1896). Fish Shellf. Immunol., 27: 100–104. https://doi.org/10.1016/j.fsi.2009.05.004
Verschuere, L., Rombaut, G., Sorgeloos, P. and Verstraete, W., 2000. Probiotic bacteria as biological control agents in aquaculture. Microbiol. mol. Biol. Rev., 64: 655-71. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC99008/, https://doi.org/10.1128/MMBR.64.4.655-671.2000
Vine, N.G., Leukes, W.D. and Kaiser, H., 2006. Probiotics in marine larviculture. FEMS Microbiol. Rev., 30: 404–427. https://doi.org/10.1111/j.1574-6976.2006.00017.x
Welker, T.L. and Lim, C.E., 2011. Use of probiotics in diets of Tilapia. J. Agric. Res. Dev., S1:14. https://doi.org/10.4172/2155-9546.S1-014
Weyant, R.S., 1996. Identification of unusual pathogenic gram-negative aerobic and facultatively anaerobic bacteria. 2nd edn. Williams and Wilkins, Baltimore.
Wouter, F.S., Bastiaens, P.I., Wirtz, K.W. and Jovin, T.M., 1998. FRET microscopy demonstrates molecular association of non-specific lipid transfer protein (nsL-TP) with fatty acid oxidation enzymes in peroxisomes. EMBO J., 17: 7179-7189. https://doi.org/10.1093/emboj/17.24.7179
Zokaeifar, H., Balcázar, J.L., Saad, C.R., Kamarudin, M.S., Sijam, K., Arshad, A. and Nejat, N., 2012. Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellf. Immunol., 33: 683–689. https://doi.org/10.1016/j.fsi.2012.05.027
To share on other social networks, click on any share button. What are these?









