Advances in Animal and Veterinary Sciences
Case Report
Report of Enterotoxaemia in Goat Kids
Kumaragurubaran Karthik*, Kaliyaperumal Manimaran, Ramasamy Bharathi, Kulasekaran Shoba
Central University Laboratory, Madhavaram Milk Colony, TANUVAS, Chennai-51, India.
Abstract | Sheep and goats are affected by various infectious diseases among which enterotoxaemia is a notable since it can cause acute infection leading to sudden death without any clinical signs. Clostridium (C) perfringens type D secretes epsilon toxin that upon absorption into the blood circulation cause enterotoxaemia. Predisposing factors such as changes in feed, damage to the gut, worm load can lead to enterotoxaemia. The present report summarizes enterotoxaemia in goat kids of two months old. Three kids out of 19 recently purchased goats died without showing any clinical signs. Necropsy findings revealed that there were hemorrhages in the intestine, brain and congested lungs. Collected samples were examined for the involvement of bacterial pathogen and C. perfringens was isolated. Molecular toxinotyping revealed that the isolate was positive for alpha and epsilon toxin genes thus confirming that the isolate as C. perfringens type D. These findings highlight the need for regular vaccination of sheep and goats to prevent enterotoxaemia.
Keywords | Enterotoxaemia, C. perfringens type D, Kids, India, Epsilon toxin, Toxinotyping
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 11, 2017; Accepted | July 09, 2017; Published | July 20, 2017
*Correspondence | K.Karthik, Central University Laboratory, Madhavaram Milk Colony, TANUVAS, Chennai-51, India.; Email: [email protected]
Citation | Karthik K, Manimaran K, Bharathi R, Shoba K (2017). Report of enterotoxaemia in goat kids. Adv. Anim. Vet. Sci. 5(7): 289-292.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.7.289.292
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Karthik et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Enterotoxaemia, also known as pulpy kidney disease or over eating disease, is an important bacterial disease of sheep and goat throughout the world, commonly seen in well-fed animals (Jemal et al., 2016). The causative organism of enterotoxaemia is a gram positive, anaerobic, spore forming and toxin producing bacilli namely Clostridium (C) perfringens type D (Uzal et al., 2014). C. perfringens is a ubiquitous organism and can cause various clinical conditions in several animal species as a result of toxin production (Niilo, 1980). C. perfringens can secrete 4 major toxins (alpha, beta, epsilon and iota) and based on these toxins the organism is typed into 5 toxinotypes as type A, B, C, D and E (Sakurai, 1995). Alpha toxin is secreted by all type of C. perfringens and epsilon toxin is indicated to be the reason for condition named as enterotoxaemia in sheep and goats (McClane et al., 2006). Factors like a sudden change of feed, high parasitic load and others that can disturb the intestinal status can predispose to enterotoxaemia (Uzal and Kelly, 1996; Lewis, 2000). Bacterial multiplication leading to epsilon toxin production in the gut of the animal, absorbed into the systemic circulation, causing increased vascular permeability in various organs. Epsilon toxin is usually produced as protoxin form, gets converted to an active form which causes the pathological lesions. Absorbed toxin can cause damage to kidneys, oedema of several organs including brain, hyperglycemia and hypertension (Garcia et al., 2013). Vaccination is commonly followed worldwide that prevents sheep and goats from enterotoxaemia still this disease occurs in several parts of the world following vaccine failure.
Acute, subacute and chronic enterotoxaemia are reported in sheep and goats of which acute infection are reported in both adult and young un-vaccinated goats while chronic cases are reported in adult vaccinated goats (Uzal and Songer, 2008). Isolation, biochemical tests, conventional toxin typing and mice neutralization assay are practiced for years but they are time consuming and laborious. Hence molecular characterization of toxins is followed in the recent years to type the isolates (Vinod Kumar et al., 2014). Enterotoxaemia is reported almost every year from various parts of India during the start of the monsoon season (Vinod Kumar et al., 2014). This report presents the bacteriological and molecular characterization of C.perfringens type D from enterotoxaemia suspected cases of recently purchased goats. Toxinotyping of the isolate has been carried out in the present study as it is essential to establish that epsilon toxin is responsible for the death of goats.
Table 1: Primers used for identification of α and ε toxin of C. perfringens
|
S. No. |
Toxin |
Gene |
Forward primer |
Reverse primer |
Product size (bp) |
Reference |
|
1 |
α |
cpa |
AAGATTTGTAAGGCGCTT |
ATTTCCTGAAATCCACTC |
1167 |
Gkiourtzidis et al. (2001) |
|
2 |
ε |
etx |
AAGTTTAGCAATCGCATC |
TATTCCTGGTGCCTTAATT |
961 |
MATERIALS AND METHODS
History
Tellicherry cross bred 19 goats (10 adults and 9 kids) were purchased one month before in an organized farm at Coimbatore district of Tamil Nadu, India. Vaccination status was not clearly known. Three kids (2 female and 1 male) of 2 months old died suddenly without any signs. These kids were well fed and were reared in mud floor. Postmortem examination revealed congestion in the lung, hemorrhages in the large intestine and generalized hemorrhage in the brain. Based on acute nature of death, clinical signs and hemorrhages in the brain tentatively the case was diagnosed as enterotoxaemia. Samples namely brain, intestinal loop with contents, liver, kidneys, heart blood swab were collected.
Bacterial Isolation
Heart blood swab, intestinal content, liver and kidney samples were processed on blood, nutrient and MacCokey agar and incubated aerobically at 37 °C for 24 hours. Intestinal contents was used for isolation and identification of anerobic bacteria in Robertson’s cooked meat (RCM) mediumand incubated at 37 °C for 24 hours. Blood agar plates were sub-cultured from the broth culture and plates were kept in McIntosh jar under anaerobic condition using anaerobic gas pack (Becton, Dickinson and Company, USA) and incubated for 24 hours. Gram staining was carried out on the pure isolates. Later biochemical tests like catalase and oxidase were performed for the isolated colonies.
Molecular Confirmation
DNA from isolated colonies were extracted by boiling a loopful of culture in 100 µL of nuclease free water for 10 min followed by freezing at -20 °C for 10 min. The content was then centrifuged at 10,000 rpm for 10 min and the supernatant was collected as the DNA. Primers reported by Gkiourtzidis et al. (2001) to detect alpha and epsilon toxin gene of C. perfringenswas used. PCR protocol was followed as per Gkiourtzidis et al. (2001). Primers used in the study are mentioned in Table 1. Agarose gel (1.5%) electrophoresis was carried out to visualize the results.
RESULTS AND DISCUSSION
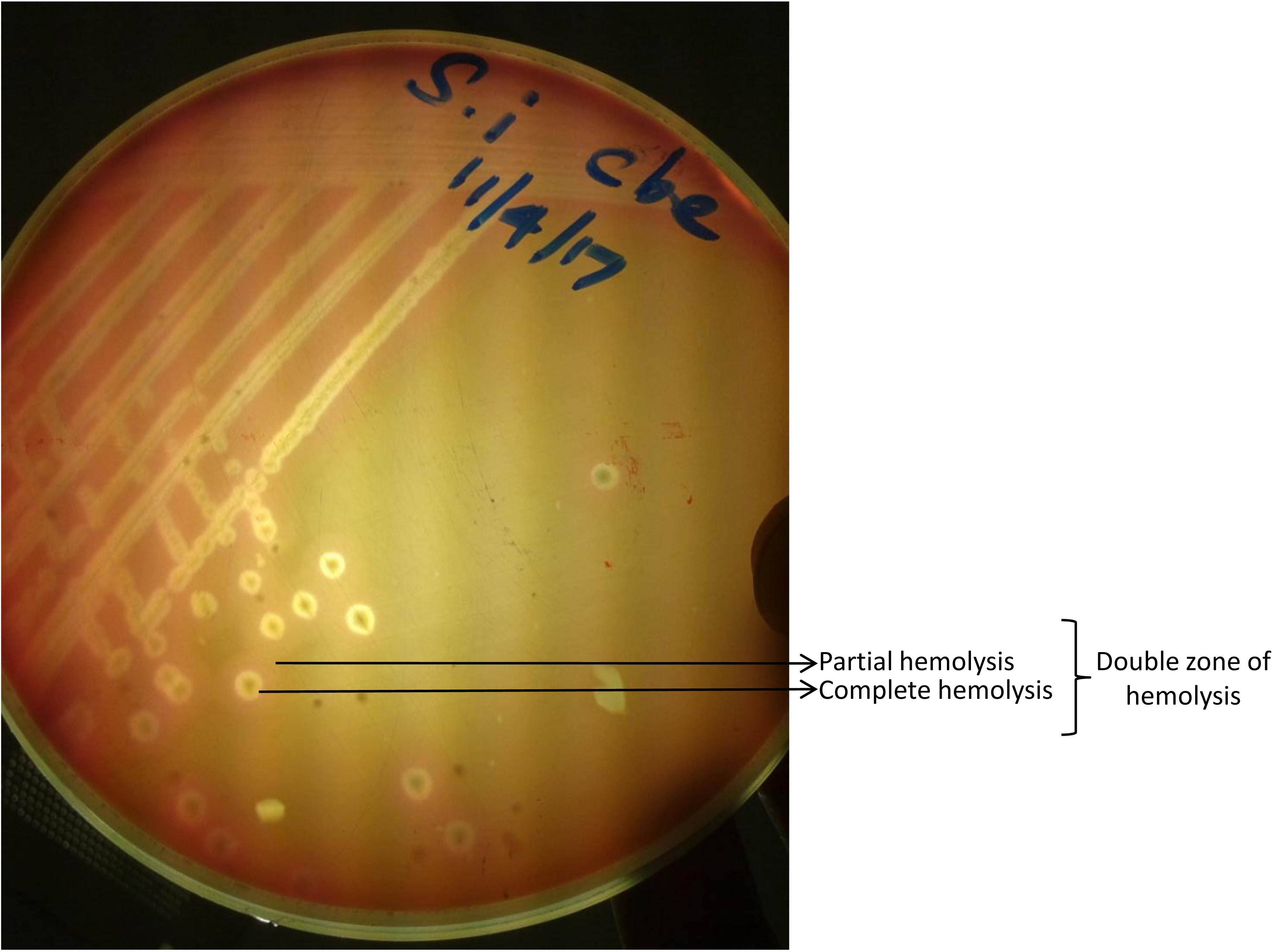
After 24 hours of incubation, there was no growth on blood, nutrient and MacConkey agar incubated aerobically while there was turbidity in RCM. Anaerobically incubated blood agar showed a double zone of hemolysis, characteristic of C. perfringens after 24 hours of incubation under anaerobic condition (Figure 1). These isolated colonies were found negative for both catalase and oxidase tests. On gram staining the organisms appeared as gram positive rods. Molecular confirmation revealed that the isolate belonged to C. perfringens type D as expected product size at 1167 bp for cpa gene and 961 bp for etx gene were visualized under UV illumination (Figure 2).
C. perfringens type D produces epsilon toxin which causes enterotoxaemia in sheep and goat. Onset of disease is sudden and the animals may die without any clinical signs (Khan et al., 2008). This report shows the acute death of goat kids without any clinical signs which were in correlation with earlier reports that acute form of enterotoxaemia is noticed in young unvaccinated goats (Uzal and Songer, 2008). C. perfringens type D is not a common pathogen of soil as like type A still it can be isolated from normal feces of sheep (Uzal and Songer, 2008). Predisposing factors like grain feeding, well fed lambs or kids and feeding on lush green pasture can cause over eating disease or enterotoxaemia (Vinod Kumar et al., 2014). In this report, the goat
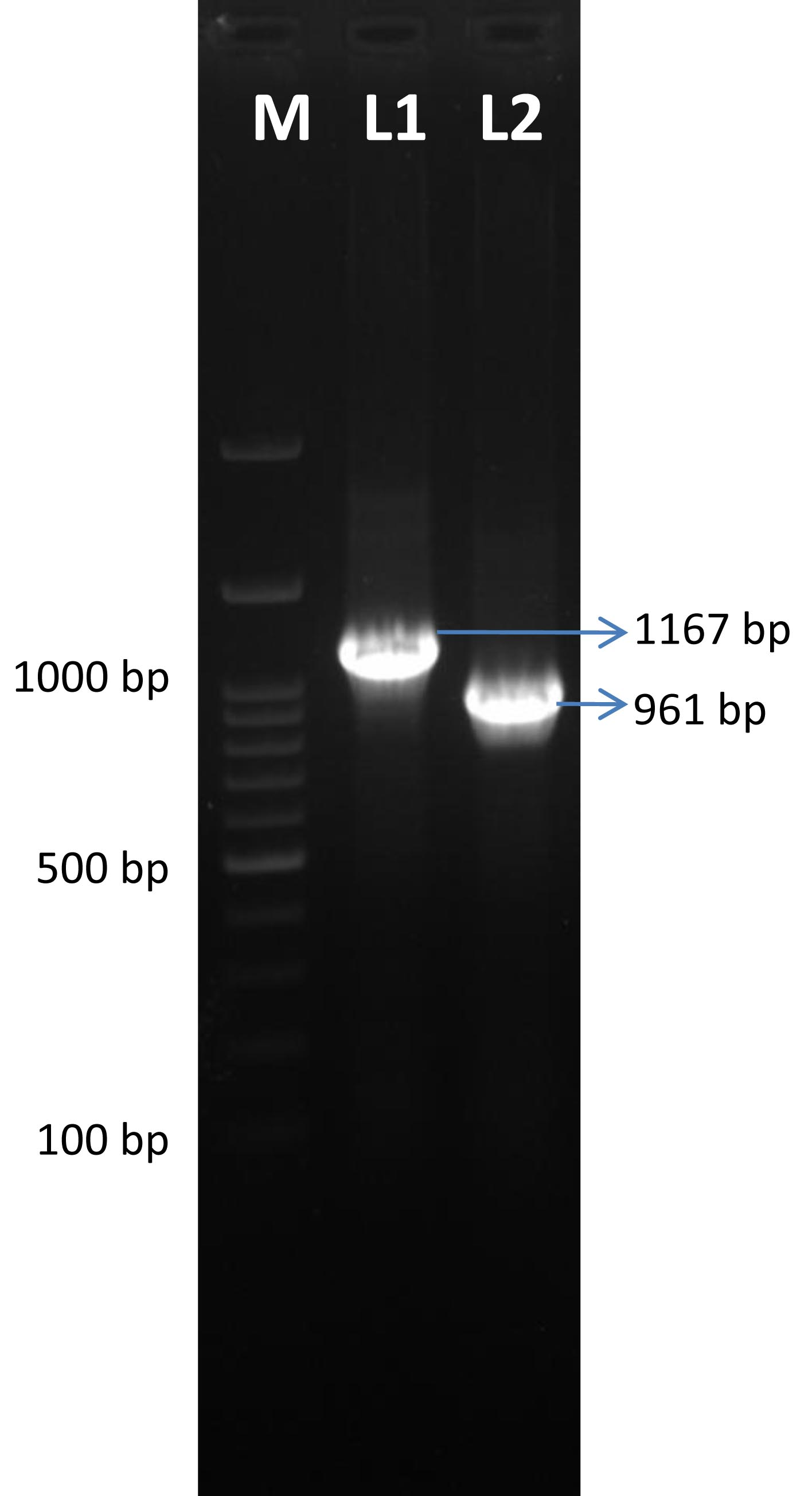
Figure 2: Agarose gel electrophoresis image of C. perfringens toxinotyping. M- 100 bp marker, L1- alpha toxin gene PCR product (1167 bp), L2- epsilon toxin gene PCR product (961 bp)
kids were purchased recently hence animals were fed with new diet and it was well fed. This may be the reason for enterotoxaemia in these kids. Though isolation and toxin neutralization assay in laboratory animals were followed earlier they are laborious and lab animal experiment involves animal ethical issues (Hadimli et al., 2012). Hence molecular toxinotyping by PCR is followed in the recent years to identify the toxin involved in the disease and also to know the type of C. perfringens involved. In the present report, the isolate was positive for cpa and etx genes which correspond to alpha and epsilon toxins. Epsilon toxin produced by C. perfringens type D is attributed for enterotoxaemia (Nasir et al., 2013) and based on the PCR assay the present isolate was typed as C. perfringens type D. Vaccination history of these newly purchased animals is not clearly known and vaccination should be followed regularly to prevent this important disease of small ruminants (Gowane et al., 2017). Periodic vaccination of small ruminants against enterotoxaemia will prevent the onset of disease thereby reducing the mortality. Thus this report summarizes the incidence of enterotoxaemia in newly purchased goat kids and warrants regular vaccination to keep enterotoxaemia in small ruminants under control.
AcKnowledgements
Authors acknowledge and thank Tamil Nadu Veterinary and Animal Sciences University.
CONFLICT OF INTEREST
There is no conflict of interest with any of the party either directly or indirectly to the content of this article.
AUTHORS CONTRIBUTION
KK, KS conducted the isolation, identification work. KK and KM carried out the molecular work. KK and RB drafted and revised the manuscript. All the authors have read and approved the final manuscript.
REFERENCES