The Effects of Discarded Cocoa (Theobroma cacao) Seed Meal Based Diets on Semen Traits, Testicular Morphometry and Histomorphology of Rabbit Bucks
Research Article
The Effects of Discarded Cocoa (Theobroma cacao) Seed Meal Based Diets on Semen Traits, Testicular Morphometry and Histomorphology of Rabbit Bucks
Luke Chukwudi Ali*, Nnanna Ephraim Ikeh, Bright Chigozie Amaefule, Amarachi Linda Obinna, Ndubuisi Samuel Machebe
Department of Animal Science, Faculty of Agriculture, University of Nigeria, Nsukka
Abstract | A 10-weeks study was carried out to evaluate the effect of dietary intake of discarded cocoa seed meal (DCSM) on reproductive characteristics of rabbit bucks. A total of 36 mixed-bred rabbit bucks of 20 weeks old and having a mean weight of 1384.34 ± 283.34g were randomly assigned to 4 treatment (T) groups of 9 rabbit bucks each replicated 3 times using a one-way analysis of variance in a completely randomized design (CRD). Water and feed were made available ad-libitum to the bucks. Diets containing 0, 15, 30 and 45% DCSM were compounded and fed to bucks in T1, T2, T3 and T4, respectively. Prior to semen collection, rabbit bucks were trained three times per week for semen collection with an artificial vagina (AV). At the end of the 10 weeks, semen collection and evaluation were carried out. Thirty six (36) rabbit bucks were slaughtered and testes collected and dissected for testicular morphometry and histomorphology evaluation. Semen colour showed milky white in all groups although T4 appeared creamy white. There were significant (P<0.01) differences among treatment groups of rabbits in all the semen traits except in semen volume. Semen pH showed significant (P<0.01) variations with semen of bucks in T4 having the highest value (8.67). Sperm motility, sperm concentration, live and normal sperms in decreased with an increase in dietary DCSM whereas non-motile, dead and abnormal sperms increased with an increase in dietary DCSM. Testicular morphometric measurements and histomorphological evaluations were similar in all treatments (P>0.05). This investigation depicts that DCSM inclusion above 15% affected semen quality negatively even though results on testicular morphometric traits and histology appeared non-significant.
Keywords | Rabbit bucks, Semen traits, Sperm characters, Testicular morphometry, Histomorphology, Discarded cocoa seed meal
Received | January 03, 2022; Accepted | March 14, 2022; Published | May 15, 2022
*Correspondence | Ali LC, Department of Animal Science, Faculty of Agriculture, University of Nigeria, Nsukka; Email: aliluke230@gmail.com
Citation | Ali LC, Ikeh NE, Amaefule BC, Obinna AL, Machebe NS (2022). The effects of discarded cocoa (Theobroma cacao) seed meal based diets on semen traits, testicular morphometry and histomorphology of rabbit bucks. Adv. Anim. Vet. Sci. 10(6): 1245-1254.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.6.1245.1254
ISSN (Online) | 2307-8316

Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Rabbits (Oryctolagus cuniculus) have the potential to fill the gap in human protein supply, particularly in developing and underdeveloped countries. They are effective feed converters, because they can withstand up to 30 percent crude fiber compared to 10 percent in most poultry species (Egbo et al., 2001). While rabbits can be raised on a forage-based diet, according to Amao and Showunmi (2016), deficient and unbalanced grass quality, as well as seasonal availability of forage, are major constraints to successful rabbit farming. Other immense potentials of rabbit include proverbial prolificacy, high growth rate, short gestation period, high genetic selection ability, relatively low cost of production, economic utilization of space and high nutritional quality (Biobaku and Oguntona, 1997; Hassan et al., 2012). In addition, the consumption of rabbit meat is free from religious and cultural biases.
Nutrition has a significant impact on the reproductive efficiency of rabbit bucks (Amao and Showunmi, 2016). Over the years, animal scientists has primarily focused on identifying less expensive alternative feedstuff that are readily available and do not compete with the dietary requirements of man (Egbunike, 1997). In a study of rabbit development on tropical feed sources, it was reported that feeding only grasses to rabbits was unsuitable due to its low digestibility of about 10% (Cheeke and Raharjo, 1988). Also, the availability of conventional feed ingredients of protein and energy sources as concentrates for rabbit diets is heavily limited. Grain production in both developed and developing countries are primarily for human consumption, resulting in extremely high feed prices (Ogbuewu et al., 2008). For instance, in developing countries like Nigeria, the key restriction to livestock development is the inconsistency in the availability of good quality feed in large amount throughout the year. As a result, the use of unconventional feed resources becomes extremely important and is promoted (Amao and Showunmi, 2016). In line with the afore-mentioned, Onifade and Tewe (1993) noted that, tropical agricultural extraction industries provide a large number of by-products with untapped feeding potential. So far, certain agricultural by-products and plants have been used in animal production with varying degrees of success.
The rabbit buck’s reproductive system produces spermatozoa that are deposited into the female genital system. Spermatozoa are formed in the testes’ seminiferous tubules and then transferred to the epididymides via the rete testes, where they are deposited until maturity (Amao et al., 2019). Spermatogenesis in rabbits takes 48 days (Amann, 1982) and includes a sequence of successive mitotic divisions, two meiotic divisions, and spermiogenesis. However, some chemical factors like reactive oxygen species or chemotherapeutic agents can interrupt this process, resulting in changes in the consistency of the sperm or the structure of the testicles (Iyama and Wilson, 2013; Chenoweth and Lorton, 2014). Cocoa and its derivatives (chocolate, cocoa cake or butter) are currently investigated as possible livestock feed ingredients.
Discarded cocoa seed meal (DCSM) are agricultural by-products that are often discarded in or around cocoa factories or communities in Southern Nigeria, resulting in environmental contamination as a result of inappropriate composting (Sukha, 2003; Nwanna and Fashae, 2010). The use of cocoa by-products as a feed option for animal feeding has alleviated the disposal problems faced by cocoa factories (Figueroa et al., 2019). When used as alternative feed ingredient, feed formulation cost is reduced, and the negative effects of climate change caused by pollution is properly mitigated.
Investigations on the benefit of the use of cocoa has revealed that it has some therapeutic effect because of their high contents of antioxidants primarily flavonoids, epicatechin, catechin, and procyanids (Visioli et al., 2000). Other contents are nitrogenous compounds, minerals, and methylxanthine (Greer et al., 2001). Even though, methylxanthine (caffeine and theobromine) are considered as anti-nutritional factors, available report has shown that it depends on the processing method and the quantity fed to the animal (Rios et al., 2003). Cocoa powder has been shown to have a variety of physiological and biological effects in vitro, such as antioxidant, anti-inflammatory, antiviral, antibacterial, vasodilatory, anticancer, and anti-ischemic effects, as well as improved endothelial cell functions, which enhance cardiovascular processes (Steinberg et al., 2003; Prochazkova et al., 2011). The feeding of dietary antioxidants to male animals have been reported to aid in minimizing the damaging effect of free radicals to sperm cells and other biological functions in the body system. Thus, chocolate (a cocoa product) has been suggested for males with fertility problems (Yildirim et al., 2014). Theobromine is of primary concern in the use of cocoa and its products as animal feed components. It has been shown to have a detrimental effect on reproduction in a variety of animal species, including rats (Tarka et al., 1981), egg laying birds (Odunsi and Longe, 1995) and rabbits (Adeyina et al., 2010).
Considering the benefit of using cocoa by products/waste as alternative feed materials, this experiment was therefore carried out to evaluate the effect of feeding discarded cocoa (Theobroma cacao) seed meal diets on semen traits, testicular morphometry and histomorphology of rabbit bucks.
Materials and Methods
Ethical approval
This study was conducted noting the recommended research ethics for scientific researches involving animal subjects: code 5.4.2 and rabbit bucks were handled according the principles of Animal Experimentation Ethics Committee University of Nigeria, Nsukka (2013).
Experimental site
This research was carried out at the Rabbit Unit of the Animal Science Teaching and Research Farm, University of Nigeria, Nsukka in Enugu State, South Eastern Nigeria. Nsukka lies in the derived Savannah region and is located at longitude 7° 21’39”E and latitude 6° 51’39”N (Ihinegbu et al., 2019) at an elevation of 456m above sea level (Onyenucheya and Nnamchi, 2018). The climate is a humid tropical setting with temperature ranging from 22.9 to 27.0 % (Phil-Eze, 2012) and a relative humidity range of 54.5 to 84.32%. In September, the peak of rainfall is 108.55mm and January and December being the lowest with no observed rainfall (Onyenucheya and Nnamchi, 2018). The light cycle of the study area was 11 hours day light.
Management of experimental animal
The discarded cocoa seed meal (DCSM) used in the diet formulation was collected in and around cocoa powder manufacturing factories in Osun, Ogun and Oyo states in Nigeria. The percentage and proximate composition of the experimental ration is presented in Table 1. A total number of thirty six (36) mixed-bred rabbit bucks of an average weight of 1384.34 ± 283.34g were randomly assigned to 4 treatments (T) diets according to the level of inclusion of discarded cocoa seed meal in the diet. Each treatment contained 9 rabbit bucks and replicated three times with each replicates containing 3 bucks. Bucks were held in individually washed and disinfected hutches with a dimension of 4ft x 2ft x 2ft and a wire-screened floor that allowed faeces and urine to pass through. Feeders and water troughs containing feed and water, respectively were provided to the bucks and allowed ad-libitum method of intake. On arrival, bucks were allowed a 1-week period to undergo acclimatization followed by an 8 weeks of feeding of the test ingredients. The rabbit bucks were also provided with equal supplemental forages plus the experimental concentrates.
Table 1: Percentage (%) and proximate composition of the experimental diets containing DCSM at various levels.
| Feedstuff |
T1 |
T2 |
T3 |
T4 |
| Maize | 39.84 | 29.48 | 19.16 | 8.84 |
| Wheat offal | 26.56 | 19.66 | 12.77 | 5.89 |
| Soya bean meal | 11.84 | 12.74 | 13.63 | 14.51 |
| Palm kernel cake | 17.76 | 19.12 | 20.44 | 21.76 |
| Cocoa seed meal | 0.00 | 15.00 | 30.00 | 45.00 |
| Limestone | 3.00 | 3.00 | 3.00 | 3.00 |
| Lysine | 0.25 | 0.25 | 0.25 | 0.25 |
| Methionine | 0.25 | 0.25 | 0.25 | 0.25 |
| Iodize salt | 0.25 | 0.25 | 0.25 | 0.25 |
| Vit-Min premixes | 0.25 | 0.25 | 0.25 | 0.25 |
|
Total proximate |
100.00 | 100.00 | 100.00 | 100.00 |
| Moisture | 5.65 | 4.80 | 4.85 | 4.85 |
| Crude protein | 15.78 | 15.92 | 16.02 | 16.51 |
| Crude fibre | 9.55 | 10.21 | 10.39 | 10.43 |
| Ash | 9.90 | 9.50 | 9.30 | 9.25 |
| Ether extract | 4.63 | 4.84 | 5.02 | 5.15 |
| Nitrogen free extract | 54.49 | 54.73 | 54.42 | 53.81 |
| Metabolizable energy (Kcal/kg) | 2570.24 | 2514.75 | 2491.69 | 2490.54 |
Semen evaluation
Thirty six (36) semen samples were collected after 10 weeks for evaluation from bucks in all the treatments. Prior to semen collection, bucks were all trained for using an artificial vagina (AV). The AV used was constructed locally with rubber rings, graduated collection tube, rubber funnel and latex inner liner (Figure 1). The consistency of the collected semen showed normal viscosity according to the method of Comhaire and Vermeulen (1995). The colour of the semen was determined by a careful visual appraisal according to Campos et al. (2014); and the volume was recorded via the use of calibrated tube attached as part of the artificial vagina. Sperm concentration was determined in a counting chamber with diluting fluid containing sodium bicarbonate, formalin and distilled water in a ratio of 1:20 using the improved Neubauer haemocytometer slide according to the method of Ochei and Kolhatkar (2000). The pH was measured using a specially treated calibrated paper blot that changes colour according to the pH of the semen (Comhaire and Vermeulen, 1995). Sperm viability was estimated using the improved one step eosin-nigrosin staining techniques according to Bjorndahl et al. (2003). Thus, live sperms will exude the eosin-nigrosin while dead sperm cells will not. Motility was determining according to World Health Organization (1992) methods. Samples from different treatments were dropped on a glass slide, covered with a slip and viewed under a microscope using x10 and x40 objectives. A minimum of 5 microscopic fields were assessed to evaluate sperm motility. The % of sperm motility was analyzed for actively motile and non-motile distinguished by the movement of the sperm cells. Sperm morphology determined by making a smear as blood film by placing a small drop of well mixed seminal fluid on a clean glass slide smearing it to a feather edge and quickly fixed (while still wet) in a cytological fixative such as 90% alcohol. The smear was stained with Giemsa. The stained smear was examined under microscope using x100 objective with immersion oil. About 100 spermatozoa with tail was counted and the percentage of abnormal forms was noted (Ochei and Kolhatkar, 2000).
Testicular morphometry of rabbit bucks
After semen collection, bucks were slaughtered using the procedure described by Blasco and Ouhayoun (1996). Through the ventral region of the abdomen, the sacrificed bucks were dissected using a sharp blade to expose both right and left testes. The organ were freed off other connective tissues leaving behind only the testis and the attached epididymis. Testes weight and paired testes weight were determined using a Mettler Toledo sensitive scale. Testes length and circumference were measured with the use of vernier caliper and a tape. The volume of the testes was determined using Archimedes’ principle of water displacement with a measuring cylinder in milliliter (ml). Relative testes weight (%) and testes density (g/ml) were calculated using the formulas described by Amao et al. (2013).
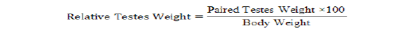
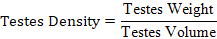
Histomorphological examination of rabbit bucks
For histomorphological test, 0.4g part of the harvested testes were collected. The samples were fixed in 10% phosphate buffered formalin for a minimum of 48 hours. The tissues were subsequently trimmed, dehydrated in four grades of alcohol (70%, 80%, 90% and absolute alcohol), cleared in three grades of xylene and embedded in molten wax. On solidifying, the blocks were sectioned, 5µm thick with a rotary microtome, floated in water bathe and incubated at 60°C for 30 minutes. The 5µm thick sectioned tissues were subsequently cleared in three grades of xylene and rehydrated in three grades of alcohol (90%, 80% and 70%). The sections were then stained with Haematoxylin for 15 minutes. Blueing was done with ammonium chloride. Differentiation was done with 1% acid alcohol before counterstaining with Eosin. Permanent mounts were made on degreased glass slides using a mountant; DPX. The prepared slides were examined with a Motic™ compound light microscope using x40 objective lens. The photomicrographs were randomly taken using a Motic™ 5.0 megapixels microscope camera at x400 magnifications. The method applied was according to Zahid et al. (2002) and Ansa et al. (2017).
Statistical analysis
All data were analyzed by a one-way analysis of variance (ANOVA) in a completely randomized design (CRD). Statistically (P<0.05) different means among treatment groups were separated according to the procedures of Duncan’s New Multiple Range Test (Duncan, 1955).
Results and Discussion
Semen traits of rabbit bucks
The results of the effect of varying dietary levels of discarded cocoa (Theobroma cacao) seed meal on semen traits of rabbit bucks are presented in Table 2.
An evaluation of the semen appearance showed that the inclusion of DCSM did not change the colour of the semen as there as there were milky white except for those in T4 that appeared creamy white. Semen pH was significantly (P<0.01) higher for the DCSM treatment groups compared with semen pH of those in the control group. Higher semen pH was shown for rabbit bucks in T4 (45% DCSM) diet and it varied from those of rabbit bucks in T2 and T3 groups. However, semen volume of rabbit bucks were similar (P<0.05) among treatment groups. Motility of spermatozoa and percentage live sperm in semen in the control, T2 and T3 were higher and did not vary (P>0.05) among treatments. Thus, at 45% DCSM dietary inclusion, the motility of sperm declined significantly (P<0.05) compared with others. Sperm concentration and percentage normal sperm were higher and similar (P>0.05) at 0 and 15% DCSM inclusion. A significant decline in these parameters were shown at 30% and 45% levels of DCSM. The percentage of non-motile and dead sperms in the semen had no significant (P>0.05) changes as the level of DCSM was increased from 0 to 30% inclusion in the diet, except at 45% where it significantly increased (P<0.05). The percentage of abnormal sperm showed no significant (P>0.05) difference at 0 and 15% DCSM, however, the number of abnormal sperm began to rise significantly from 30% to 45% DCSM dietary inclusion (P<0.05).
Testicular morphometry and histomorphology of rabbit buck
The results of the effect of varying dietary levels of discarded cocoa (Theobroma cacao) seed meal on the testicular morphometry of rabbit bucks are presented in Table 3. The results on testicular morphometry of rabbit bucks fed DCSM diet were not significantly (P>0.05) different among the treatment groups except in T4 with spermatogonial disruption. Figure 2 presents the micrograph of the histomorphology of rabbit buck testes fed varying dietary levels of discarded cocoa (Theobroma cacao) seed meal. It showed that no testicular histomorphological differences were observed among different treatments expose to DCSM. The sections of the testes presented in all the experimental treatments showed normal testicular histomorphology. Thus, the magnified sections of the testes
Table 2: Semen evaluation of rabbit bucks fed discarded cocoa seed meal based diets.
| Parameters |
T1 (0% DCSM) |
T2 (15% DCSM) |
T3 (30% DCSM) |
T4 (45% DCSM) |
P. value |
| Semen appearance | Milky white | Milky white | Milky white | Creamy white | |
| Semen pH |
6.17 ± 0.17c |
7.00 ± 0.00b |
6.83 ± 0.17b |
8.67 ± 0.33a |
0.000** |
| Semen volume (ml) | 0.83 ± 0.09 | 0.77 ± 0.07 | 1.10 ± 0.15 | 0.77 ± 0.12 |
0.190NS |
| Sperm motility (%) |
76.67 ± 3.33a |
76.67 ± 3.33a |
70.00 ± 0.00a |
46.67 ± 3.33b |
0.000** |
|
Sperm Con. (x106/ml) |
84.67 ± 9.26a |
89.33 ± 5.21a |
57.67 ± 1.45b |
39.67 ± 9.35b |
0.003** |
| Non-motile sperm (%) |
23.33 ± 3.33b |
23.33 ± 3.33b |
30.00 ± 3.33b |
53.33 ± 5.77a |
0.002** |
| Live sperm (%) |
86.67 ± 3.33a |
86.67 ± 3.33a |
80.00 ± 0.00a |
53.33 ± 3.33b |
0.000** |
| Dead sperm (%) |
13.33 ± 3.33b |
13.33 ± 3.33b |
20.00 ± 0.00b |
46.67 ± 3.33a |
0.000** |
| Normal sperm (%) |
76.67 ± 3.33a |
76.67 ± 3.33a |
63.33 ± 3.33b |
36.67 ± 3.33c |
0.000** |
| Abnormal sperm (%) |
23.33 ± 3.33c |
23.33 ± 3.33c |
36.67 ± 3.33b |
63.33 ± 3.33a |
0.000** |
abc: Means ± SEM on the same row with different superscripts are significantly different (P ≤ 0.05 or P ≤ 0.01); Con.: Concentration.
Table 3: Testicular morphometry of rabbit bucks fed discarded cocoa seed meal based diets.
| Parameters |
T1 (0% DCSM) |
T2 (15% DCSM) |
T3 (30% DCSM) |
T4 (45% DCSM) |
P. Value |
| Live weight (kg) | 2.10 ± 0.10 | 2.00 ± 0.20 | 2.05 ± 0.15 | 1.8 ± 0.20 |
0.641NS |
| Testicular characteristics | |||||
| Right testis length (cm) | 3.20 ± 0.00 | 3.25 ± 0.05 | 3.20 ± 0.10 | 3.10 ± 0.20 |
0.824NS |
| Left testis length (cm) | 3.30 ± 0.00 | 3.25 ± 0.05 | 3.25 ± 0.15 | 3.10 ± 0.20 |
0.724NS |
| Right testis circumference (cm) | 3.90 ± 0.30 | 3.95 ± 0.35 | 4.15 ± 0.05 | 3.65 ± 0.05 |
0.565NS |
| Left testis circumference (cm) | 3.95 ± 0.25 | 4.05 ± 0.35 | 4.20 ± 0.00 | 3.65 ± 0.15 |
0.465NS |
| Right testis weight (g) | 2.75 ± 0.25 | 2.95 ± 0.25 | 2.45 ± 0.05 | 2.10 ± 0.40 |
0.269NS |
| Left testis weight (g) | 2.55 ± 0.15 | 2.35 ± 0.35 | 2.60 ± 0.10 | 2.10 ± 0.50 |
0.696NS |
| Paired testes weight (g) | 5.25 ± 0.35 | 4.80 ± 0.60 | 5.25 ± 0.05 | 4.25 ± 0.85 |
0.578NS |
| Right testis volume (ml) | 3.00 ± 0.00 | 3.00 ± 0.00 | 3.00 ± 0.00 | 2.25 ± 0.50 |
0.479NS |
| Left testis volume (ml) | 2.50 ± 0.50 | 2.50 ± 0.50 | 3.50 ± 0.05 | 2.50 ± 0.50 |
0.479NS |
| Paired testes volume (ml) | 5.50 ± 0.50 | 5.50 ± 0.50 | 6.50 ± 0.50 | 5.00 ± 0.00 |
0.242NS |
| Relative testes weight (%) | 0.25 ± 0.01 | 0.24 ± 0.01 | 0.26 ± 0.02 | 0.23 ± 0.02 |
0.586NS |
| Testes density (g/ml) | 0.96 ± 0.03 | 0.87 ± 0.03 | 0.81 ± 0.07 | 0.85 ± 0.17 |
0.747NS |
abc: Means ± SEM on the same row with different superscripts are significantly different (P ≤ 0.05 or P ≤ 0.01).
showed that a normal architecture were observed in control and treated groups and a clear and well separated seminiferous tubules surrounded by the basement membrane were shown. The only exception is found in T4 containing 45% DCSM with some degrees of disruption of sperm cells.
The current study was carried out to determine various significant effect of DCSM on the reproductive aspect of rabbit bucks especially on semen traits, testicular morphometry and histomorphology. Results on semen evaluation showed that the test ingredients had various effects on the semen quality indices. The similar milky whitish appearance observed across T1 to T3 are indications that DCSM up to 30% inclusion did not cause any malfunctioning or inflammation of the reproductive tract or the accessory sex glands (Banerjee, 2010). The only difference was experienced in T4 with a creamy whitish appearance which might be as a result of the dark colour of the DCSM. Kostic (1997) stated that the alkalization process during cocoa processing will cause a darker colour of cocoa powder than the natural state. Thus, Jon (2020) reported that dietary changes may alter the semen colour in some cases. Semen pH and volume recorded in this work are within the normal range according to International Rabbit Reproduction Group (2005) and similar to values observed by Ajuogu et al. (2018) in rabbit bucks semen. The increase in semen pH with an increase in DCSM is in line with the report of Li et al. (2012) that the nature of increase in pH can be explained by the fact that the manufacturing process of cocoa powder involves an alkalization process which generally increases the pH of natural product from 5.2 and 5.6 to between 6.8 and 8.0 depending on the condition of alkalization. The non-significant differences observed for semen volume in this research is the same as those reported by Ojezele et al. (2016).
The results of sperm concentration, active, live and normal sperms showed that with an increasing concentration of DCSM above 15%, there exist a negative effect on the semen quality. Parameters for poor semen traits (non-motile, dead and abnormal sperm cells) increased with an increase in DCSM above 15% inclusion level. Thus, this is an indication that DCSM should be included in a minimal level to avoid detrimental effect on the semen quality. The results in this study is similar with the findings obtained by Okunola et al. (2017). According to Munier (2018), the addition of fermented cocoa product up to 20% level led to a decrease in both microscopic and macroscopic semen quality of goat. In addition, our result does not corroborate what Collodel et al. (2014) reported, that both chocolate (cocoa product) and propolfenol exerted a protective effect on spermatogenesis and sperm characteristics of rabbit bucks. Spermatogenesis is under the control of the pituitary-hypothalamic-gonadal axis, it is possible that bioactive constituents bound in cocoa powder might have debilitating effects at a certain level on this axis or the testis (Friedman et al., 1979; Minji et al., 2015). It has been reported that theobromine in cocoa powder or the extract showed a negative effect on the sertoli cell leading to failed release of spermatids (Wang et al., 1992), reduction in the prostate and seminal vesicle (Funabashi et al., 2000), hyperplasia of the atrophied Leydig cells (Weinberger et al., 1978), decrease in the fluid volume of the seminiferous tubules (Wang and Waller, 1994) in rats.
Results on testicular morphometry showed no significant (P>0.05) variations across treatment groups. Similar non-significant (P>0.05) observations were obtained by Amao and Showunmi (2016) and Velasquez-Pereira et al. (1998). Results from this research contradicts the significant (P<0.05) different results reported by Ozung et al. (2019) on rabbit bucks reproductive tract morphometry on some parameters after dietary cocoa husk meal intake. Amao et al. (2013) also reported significant (P<0.05) differences when rabbit bucks were fed diets containing neem (Azadirachta Indica A. Juss) plant meal. Although the present study observed no significant (P>0.05) differences in all parameters, but are within similar range of values reported by Abu et al. (2013); Olarotimi et al. (2015); Abu et al. (2016); Amao et al. (2019); Ozung et al. (2019). According to Coulter (1980), testicular circumference is positively related to testicular weight for species like buck and ram and this report is similar with result of this study. Furthermore, Ahemen et al. (2016) reported that, testis length is among the indicators for the assessment of spermatogenesis, also Perry and Petterson (2001) and Akpa et al. (2013) noted that, length of the testis is a measure of the size of that testis and all these are positively related to sperm production.
The slight non-significant increased values observed in this study might be as a result of cocoa polyphenols. Reactive oxygen species (ROSs’) are important components in reproductive mechanisms and the production of ROS is a normal physiological process in several organs including the testes; however, excessive production can appear deleterious to the testis or male fertility (Akiyama, 1999; Al-Gubory et al., 2010). Thus, polyphenols as a strong antioxidant tends to ameliorate oxidative damages of ROS (Rucker, 2009). From all indications, the non-significant (P>0.05) differences reported based on the effect of DCSM on the reproductive organ of rabbit buck testis, probably may not have been observed morphometrically at the duration of this study. The effect of DCSM did not cause hypertrophy or hyperplasia in the testis of rabbit bucks throughout the duration of this study and Zar (1996) stated that increased sizes and weights of body organs are as a result of hypertrophy and hyperplasia. Gans et al. (1980) and Tarka et al. (1981) reported that the effect of theobromine on the reproductive organs of male animals were found to be both time and dose dependent.
The photomicrograph on the testes histology obtained were similar with the findings of Abdul-Rahman (2015). The anatomy and histology of the reproductive systems of rabbits according to Herrera et al. (2014) and Zamora et al. (2014) showed a similar morphology as the ones obtained in this study. Uzochukwu (2016), reported a no testicular histomorphological differences when African catfish (Clarias garienpinus) were fed diets containing cocoa bean meal. In other research, non-pathological changes were reported by Shinkut (2015) after feeding rabbit buck with dietary supplemental garlic (Allium sativum). At 200mg/kg of diet, it was reported that unsweetened natural cocoa powder had no toxic effect on the testicular histology of Sprague-Dawley rats (Asiedu-Gyekye et al., 2016). Conversely, Oguike et al. (2019) reported a varying degree of degeneration in the histology of rabbit bucks fed Aspilia africana leaf meal when compared to the control group; and similar observation was also reported in wistar rats by Asuquo et al. (2015). This outcome probably might be as a result of theobromine inherent in cocoa powder which is capable of causing debilitating effect on the testis (Smit, 2011). This ideology is supported by Eteng et al. (2005); European Food Safety Authority (2008) and Park et al. (2015).
Conclusions and Recommendations
It was concluded from this study that feeding rabbit bucks DCSM diet suppressed semen quality even though results from testicular morphometry showed no significant (P>0.05) variations. At 45% inclusion of DCSM, spermatogonial disruption was also reported. Theobromine inherent in DCSM might be the resulting factor leading to these poor result. Theobromine effect could be minimized by practicing detheobromination techniques in other to reduce its content in any sample of cocoa powder. It is recommended that dietary inclusion of DCSM in rabbit bucks feeding must not exceed 15% inclusion level.
Acknowledgements
The authors would like to acknowledge their affiliate university and the anonymous reviewers for their constructive and immense contributions towards the improvement of the manuscript. We also thank Oguejiofor, C. Innocent and Ukwueze, O. Chidera for their contributions during the fieldwork.
Novelty Statement
To the best of our knowledge, this study is the first to decipher the effects of DCSM (Theobroma cacao) based diet in rabbit bucks with interest on semen, sperm, testicular morphometry and histomorphological traits in a tropical environment, and therefore can be used as alternative feedstuff.
Author’s Contribution
Ali LC: Conceptualized the work, prepared first and final drafts. Ikeh NE: Statistical analysis and literature review. Amaefule BC: Literature review and data collection. Obinna AL: Data collection and sorting. Machebe NS: Reviewed manuscript and supervised the field work.
Conflict of interest
The authors have declared no conflict of interest.
References
Abdul-Rahman AI (2015). Comparative Histological studies of the kidney, liver and testes of the adult male domestic and wild rabbits (Oryctolagus cuniculus) in Saudi Arabia. J. Am. Sci., 11(12): 270 - 275.
Abu AH, Ahemen T, Ikpechukwu P (2013). The testicular morphometry and sperm quality of rabbit bucks fed graded levels of Moringa Oleifera Leaf Meal (MOLM). Agrosearch, 13(1): 49-56. https://doi.org/10.4314/agrosh.v13i1.5
Abu AH, Okwori IA, Ahemen T, Ojabo LD (2016). Testicular and epididymal characteristics of rabbit bucks fed Tephrosia bracteolata leaf meal. Int. J. Livest. Res., 6(11): 74-82. https://doi.org/10.5455/ijlr.20160829021232
Adeyina AO, Apata DF, Annongu AA, Olatunde OA, Alli OI, Okupke KM (2010). Performance and physiological response of weaner rabbits fed hot water treated cocoa bean shell based diet. Res. J. Anim. Vet. Sci., 5: 53-57.
Ahemen T, Abu AH, Akuba JO (2016). Effect of Gmelina arborea leaf meal on sperm production and sperm reserves in rabbit bucks. Int. J. Livest. Res., 6: 98-104. https://doi.org/10.5455/ijlr.20160319123320
Ajuogu PK, Herbert U, Ibeh PC, Nodu MB, Ukpabio UH, Onyegbule CG, Akintola AO (2018). Semen quality characteristics and testosterone levels of rabbit bucks fed Costus afer leaf. Afr. J. Biotechnol., 17(2): 24-28. https://doi.org/10.5897/AJB2016.15833
Akiyama M (1999). In vivo scavenging effect of ethylcysteine on reactive oxygen species in human semen. Nippon Hinyokika Gakkai Zasshi, 90: 421-428. https://doi.org/10.5980/jpnjurol1989.90.421
Akpa GN, Ambali AL, Suleiman IO (2013). Relationships between semen cation concentration, semen characteristics, testicular measurement and body conformation trait in red Sokoto goat. Nat. Sci., 11(7): 94-99.
Al-Gubory KH, Fowler PA, Garrel C (2010). The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell. Biol., 42: 1634-1650. https://doi.org/10.1016/j.biocel.2010.06.001
Amann RP (1982). Use of animal models for detecting specific alterations in reproduction. Fundam. Appl. Toxicol., 2: 13-26. https://doi.org/10.1093/toxsci/2.1.13
Amao EA, Oladipo AO, Sokunbi OA (2013). Testicular characteristics and daily sperm production of rabbit bucks fed diets containing neem (Azadirachta indica A. Juss) rind meal. Greener J. Agric. Sci., 3(8): 623-627. https://doi.org/10.15580/GJAS.2013.3.040213554
Amao EA, Sokunbi OA, Agbaye FP, Adeoti TM (2019). Testicular characteristics and daily sperm production of male rabbits placed on varying levels of pawpaw seed (Carica papaya) meal. World J. Agric. Res., 7(4): 158-160.
Amao OA, Showunmi KA (2016). Reproductive characteristics of rabbit bucks fed diet containing raw or fermented cottonseed cake. Br. Biotechnol. J., 10(3): 1-7. https://doi.org/10.9734/BBJ/2016/15383
Ansa AA, Akpere O, Imasuen JA (2017). Semen traits, testicular morphometry and histopathology of cadmium-exposed rabbit bucks administered methanolic extract of Phoenix dactylifera fruit. Acta Sci. Anim. Sci., 39(2): 207-215. https://doi.org/10.4025/actascianimsci.v39i2.32858
Asiedu-Gyekye IJ, Frimpong-Manso S, N’guessan BB, Seidu MA, Osei-Prempeh P, Boamah DK (2016). Macro- and micro elemental composition and toxicity of unsweetened natural cocoa powder in sprague-dawley rats. J. Toxicol., 2016: 1-11. Article ID 4783829. https://doi.org/10.1155/2016/4783829
Asuquo OR, Eluwa MA, Mesembe OE, Ekanem TB (2015). Anti-spermatogenic activity of Aspilia africana methanol leaf extract in male Wistar rats. Br. J. Med. Med. Res., 6(4): 415-422. https://doi.org/10.9734/BJMMR/2015/12144
Banerjee GC (2010). A textbook of animal husbandry: Mechanisms of reproduction. 8th Edition. Oxford & IBH Publishing Company.
Biobaku WO, Oguntona EB (1997). The effects of feeding multi nutrient mini blocks and pelleted diet on the growth of rabbits. Nig. J. Anim. Prod., 24(2): 147-149. https://doi.org/10.51791/njap.v24i2.2338
Bjorndahl L, Soderlund I, Kvist U (2003). Evaluation of the one‐step eosinnigrosin staining technique for human sperm vitality assessment. Hum. Reprod., 18: 813–816. https://doi.org/10.1093/humrep/deg199
Blasco A, Ouhayoun J (1996). Harmonization of criteria and terminology in rabbit meat research. World Rabbit Sci., 4: 93–99. https://doi.org/10.4995/wrs.1996.278
Campos ACN, Gadelha CRF, Guerreiro MEF, Pereira ES, Lima ICS, Linard MAB, Meneses HM, Castelo-Branco KF, Estevam FNL (2014). Male rabbit reproductive physiology. Stand. Res. J. Agric. Sci., 2: 120-128
Cheeke PR, Raharjo C (1988). Evaluation of tropical forages and agricultural by-products as feed for Rabbits. In: Intensive systems for Animal production and Renewable Energy with Tropical Resources, (Editors: Preston TR, Rosales M) CIPAV: Cali. Colombia, 2: 33-42.
Chenoweth PJ, Lorton S (2014). Animal andrology: Theories and applications. Wallingford, Oxfordshire, UK, CABI. https://doi.org/10.1079/9781780643168.0000
Collodel G, Moretti E, Del Vecchio MT, Biagi M, Cardinali R, Mazzi L, Brecchia G, Maranesi M, Manca D, Castellini C (2014). Effect of chocolate and propolfenol on rabbit spermatogenesis and sperm quality following bacterial lipopolysaccharide treatment. Syst. Biol. Reprod. Med., 60(4): 217–226. https://doi.org/10.3109/19396368.2014.911392
Comhaire F, Vermeulen L (1995). Human semen analysis. Hum. Reprod. Update, 1(4): 343-362. https://doi.org/10.1093/humupd/1.4.343
Coulter GH (1980). Testicular development: Its management and significance in young beef bulls. Proc. 8th Tech Conf. Nat. Assoc. Anim. Breeders. pp. 106-111.
Duncan DB (1955). Multiple range and multiple F tests. Biometrics, 11: 1-41. https://doi.org/10.2307/3001478
Egbo ML, Doma UD, Lacdacks AB (2001). Characteristics of small-scale rabbit production and management in Bauchi Metropolis. Proceedings of the 26th annual conference of Nigeria Society for Animal Production (NSAP). pp. 18-21.
Egbunike GN (1997). What is Animal science and how can Nigeria get out of Malnourishment? In: Proc. 2nd Ann. Confr. Animal Sci. Assoc. Nigeria. Sept. 16-17th, Ikeja Lagos, Pp. 1 - 12.
Eteng MU, Eyong EU, Ifere GO, Chukwuemeka N (2005). Theobromine induced seminiferous tubular lesion with elevated serum testosterone levels in male wistar rats. Biokemistri, 17(2): 123-128. https://doi.org/10.4314/biokem.v17i2.32597
European Food Safety Authority (2008). Scientific opinion of the panel on contaminants in the food chain on a request from the European commission on theobromine as undesirable substances in animal feed. EFSA J., 725: 1-66.
Figueroa KHN, Garcia NVM, Campos-Vega R (2019). Cocoa by-products. Food wastes and by-products: Nutraceutical and health potential. First edition. Edited by Rocio Campos-Vega, B. Dave Oomah, and Haydé Azeneth Vergara-Castañeda. Published by John Wiley & Sons Ltd.
Friedman L, Weinberger MA, Farber TM, Moreland FM, Peters EL, Gilmore CE, Khan MA (1979). Testicular atrophy and impaired spermatogenesis in rats fed high levels of the methylxanthines caffeine, theobromine, or theophylline. J. Environ. Pathol. Toxicol., 2: 687–706.
Funabashi H, Fujioka M, Kohchi M, Tateishi Y, Matsuoka N (2000). Collaborative work to evaluate toxicity on male reproductive organs by repeated dose studies in rats. 22 Effects of 2- and 4-week administration of theobromine on the testis. J. Tox. Sci., 25: 211-221. https://doi.org/10.2131/jts.25.SpecialIssue_211
Gans JH, Korson R, Cater MR, Ackerly CC (1980). Effects of short-term and long term theobromine administration to male dogs. Toxicol. Appl. Pharmacol., 53: 481-496. https://doi.org/10.1016/0041-008X(80)90360-9
Greer F, Hudson R, Ross R, Graham T (2001). Caffeine ingestion decreases glucose disposal during a hyperinsulinemiceuglycemic clamp in sedentary humans. Diabetes, 50: 2349-2354. https://doi.org/10.2337/diabetes.50.10.2349
Hassan HE, Elamin KM, Yousif IA, Musa AM, Elkhairey MA (2012). Evaluation of body weight and some morphometric traits at various ages in local rabbits of Sudan. J. Anim. Sci. Adv., 2(4): 407-415.
Herrera JA, Hernandez JA, Ordaz RI, Epinosa JH, Martinez MP (2014). Histometric parameters of uterus and vagina in Breeder female rabbits on the day after weaning according to the ordinal number of parturition. Int. J. Morphol., 32(2): 732-737. https://doi.org/10.4067/S0717-95022014000200058
Ihinegbu C, Nnadi GS, Madu IA (2019). Analysis of economic benefits of flooding in Alor Uno Community, Nsukka, Enugu State. Int. J. Geogr. Environ. Manag., 5(2): 76-90.
International Rabbit Reproduction Group (2005). Guidelines for the handling of rabbit bucks and semen. World Rabbit Sci., 13(2): 71-91. https://doi.org/10.4995/wrs.2005.527
Iyama T, Wilson DM (2013). DNA repair mechanisms in dividing and non-dividing cells. DNA Repair (Amst), 12: 620-636. https://doi.org/10.1016/j.dnarep.2013.04.015
Jon J (2020). Why does semen colour change and what does it mean? An online article retrieved 24th February, 2021 from https://www.medicalnewstoday.com/articles/semen-color.
Kostic MJ (1997). Cocoa alkalization. The manufacturing confectioner. Presented at the pennsylvania manufacturing confectioners’ association 51st Annual Production Conference, June.
Li Y, Feng Y, Zhu S, Luo C, Ma J, Zhong F (2012). The effect of alkalization on the bioactive and flavor related components in commercial cocoa powder. J. Food Compos. Anal., 25(1): 17-23. https://doi.org/10.1016/j.jfca.2011.04.010
Minji P, Yuri C, Hyeonhae C, Ju-Yearn Y, Jaesook R (2015). High doses of caffeine during the peripubertal period in the rat impair the growth and function of the testis. Int. J. Endocrinol., 2015: 9 pages. Article ID 368475. https://doi.org/10.1155/2015/368475
Munier FF (2018). Effect of fermented cocoa pod husk level in concentrate on Etawah Grade semen quality. IOP Conf. Series: Earth and Environmental Science, 119. 01235. https://doi.org/10.1088/1755-1315/119/1/01203.
Nwanna LC, Fashae OF (2010). Use of discarded cocoa bean meal as a source of dietary energy for the production of African catfish (Clarias gariepinus, Burchell 1822). World Aquacult., 41(2): 12-16.
Ochei J, Kolhaktar A (2000). Medical laboratory sciences, theory and practice. Tata McGraw-Hill Publishing Company Limited, New Delhi. pp. 276-287.
Odunsi AA, Longe OG (1995). Cocoa bean cake in poultry diets I. Chemical composition and nutritive value of cocoa bean cake in pullet chick diets. J. App. Anim. Res., 7: 91-97. https://doi.org/10.1080/09712119.1995.9706055
Ogbuewu IP, Okoli IC, Iloeje MU (2008). Serum biochemical evaluation and organ weight characteristics of buck rabbits fed graded levels of neem (Azadirachta indica) leaf meals diets. Priory Lodge Education Limited.
Oguike MA, Onuta ST, Amaduruony W, Akpan IU (2019). Impact of Aspilia africana on semen and testicular characteristics of rabbit bucks. J. Adv. Agric. Technol., 6(2): 144-149. https://doi.org/10.18178/joaat.6.2.144-149
Ojezele MO, Erhirhie EO, Arojojoye OA (2016). Effects of Viscum album (mistletoe) from three host plants (cocoa, kola and coffee) on semen quality of wistar albino rats. Chem. Int., 2(1): 109-113.
Okunola AA, Babatunde EE, Temitope OJ, Oludare OE (2017). Assessment of Environmental Contamination by Wastewater from a Cocoa Processing Industry Using Genetic and Reproductive Biomarkers. J. Toxicol. Risk Assess 3. http:s//doi.org/10.23937/2572-4061.1510008.
Olarotimi OJ, Sokunbi OA, Abdur-Rahman A (2015). Determination of daily sperm production (DSP) in rabbit (Oryctolagus cuniculus) bucks using testicular parameters. Greener J. Agric. Sci., 5(4): 141-148. https://doi.org/10.15580/GJAS.2015.4.070715086
Onifade AA, Tewe OO (1993). Alternative tropical feed resources in rabbit diets, growth performance, diet digestibility and blood composition. World Rabbit Sci., 1: 17-24. https://doi.org/10.4995/wrs.1993.191
Onyenucheya CO, Nnamchi HC (20118). Diurnal and annual mean weather cycles over Nsukka, Nigeria during 2010/2011. Nigerian J. Technol., 37(2): 519-524. https://doi.org/10.4314/njt.v37i2.31
Ozung PO, Kennedy OO, Ubua JA, Agiang EA (2019). Dietary cocoa pod husk meal could influence the reproductive tract morphometry of rabbits. East Afr. Sch. J. Agric. Life Sci., 2(1): 26-30.
Park M, Choi Y, Choi H, Yim J, Roh J (2015). High doses of caffeine during the peripubertal period in the rat impair the growth and function of the testis. Int. J. Endocrinol., 2015: 9 pages. Article ID 368475. https://doi.org/10.1155/2015/368475
Perry G, Petterson D (2001). Determining reproduction fertility in herd bulls. University of Missouri Agricultural Publication G, USA. pp. 1-8.
Phil-Eze PO (2012). The influence of elevation and aspect on plant species diversity in a tropical landscape of Nsukka plateau in Nigeria. Trop. Built Environ. J., 1(3): 255-266.
Procházková D, Boušová I, Wilhelmová N (2011). Antioxidants and prooxidants properties of flavonoids. Fitoterapia, 82: 513-523. https://doi.org/10.1016/j.fitote.2011.01.018
Rios LY, Gonthier MP, Remesy C, Mila I, Lapierre C, Lazarus SA, Williamson G, Scalbert A (2003). Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am. J. Clin. Nutr., 77: 912-918. https://doi.org/10.1093/ajcn/77.4.912
Rucker R (2009). Nutritional properties of cocoa. In: Chocolate: History, culture, and heritage. Edited by Grivetti and Shapiro. John Wiley & Sons, Inc.
Shinkut M (2015). Semen characteristics, gonadal sperm reserves and haematological parameters of rabbit bucks fed diets supplemented with Allium sativum (Garlic). A dissertation submitted to the school of postgraduate studies, in partial fulfillment of the requirements for the award of the degree of Master of Science in Theriogenology, Ahmadu Bello University, Zaria.
Smit HJ (2011). Theobromine and the pharmacology of cocoa. In Methylxanthines; Springer: Berlin, Germany; Heidelberg, Germany, pp. 201-234. https://doi.org/10.1007/978-3-642-13443-2_7
Steinberg FM, Bearden MM, Keen CL (2003). Cocoa and chocolate flavonoids: Implications for cardiovascular health. J. Am. Diet Assoc., 103: 215-223. https://doi.org/10.1053/jada.2003.50028
Sukha DA (2003). Potential value added products from Trinidad and Tobago cocoa. In Proceedings of the Revitalization of the Trinidad and Tobago cocoa industry. Targets, Problems and Options, St. Augustine, FL, USA, 20th September; pp. 69-73.
Tarka SM, Zoumas BL, Gans JH (1981). Effects of continuous administration of dietary theobromine on rat testicular weight and morphology. Toxicol. Appl. Pharmacol., 58: 76-82. https://doi.org/10.1016/0041-008X(81)90117-4
Uzochukwu IE (2016). Growth, gonadal development, and blood profile in African catfish (Clarias gariepinus, Burchell 1822) fed diets containing cocoa bean meal. Research project report submitted to the department of animal science, faculty of agriculture, University of Nigeria, Nsukka in partial fulfilment of the requirements for the award of Master’s degree in animal reproductive physiology.
Velasquez-Pereira J, Chenoweth PJ, McDowell LR, Risco CA, Williams SN, Staples CR (1998). Reproductive effects of feeding gossypol and vitamin E to bulls. J. Anim. Sci., 76: 2894-2904. https://doi.org/10.2527/1998.76112894x
Visioli F, Caruso D, Galli C, Viappiani S, Galli G, Sala A (2000). Olive oils rich in natural catecholic phenols decrease isoprostane excretion in humans. Biochem. Biophys. Res. Communs., 278: 797-799. https://doi.org/10.1006/bbrc.2000.3879
Wang Y, Waller DP (1994). Theobromine toxicity on Sertoli cells and comparison with cocoa extract in male rats. Toxicol. Lett., 70: 155–164. https://doi.org/10.1016/0378-4274(94)90159-7
Wang Y, Waller DP, Sinha Hikim AP, Russell LD (1992). Reproductive toxicity of theobromine and cocoa extract in male rats. Reprod. Toxicol., 6: 347-353. https://doi.org/10.1016/0890-6238(92)90198-3
Weinberger MA, Friedman L, Farber TM, Moreland FM, Peters EL, Gilmore CE, Khan MA (1978). Testicular atrophy and impaired spermatogenesis in rats fed high levels of the methylxanthines caffeine, theobromine, or theophylline. J. Environ. Pathol. Toxicol., 1: 669-688.
World Health Organization, WHO (1992). WHO labouratory manual for the examination of human semen and sperm-cervical mucus interaction. 3rd Ed., Cambridge University Press, Cambridge.
Yildirim E, Çınar M, Yalçınkaya I, Ekici H, Atmaca N, Güncüm E (2014). Effect of cocoa butter and sunflower oil supplementation on performance, immunoglobulin, and antioxidant vitamin status of rats. Bio. Med. Res. Int., 60: 65-75. https://doi.org/10.1155/2014/606575
Zahid IA, Lohdi LA, Rehman N, Akhtar MS (2002). Effects of gossypol on micrometry of teddy male goats. Pak. Vet. J., 22(3): 101-104.
Zamora EJ, Felipe-Pérez YE, Velázquez CS, Valladares CB, Fajardo MR, Quezada-Barrera KC, Cano TR, Perez SL, Diaz GB (2014). Histological description of the rabbit (Oryctolagus cuniculus) epididymis and testicles. Department of Animal Reproduction, Faculty of Veterinary Medicine and Zootechnics, Autonomous University of the State of Mexico. The Cerrillo Piedras Blancas, CP 50200, Toluca, México. pp. 205-219.
Zar JH (1996). Bio-statistical analysis. Vol. 3, Prentice-Hall, New Jersey, pp: 123-129.
To share on other social networks, click on any share button. What are these?





