Strongyliasis Occurs in Epidemic Proportion amongst other Nematodiasis and Cestodiasis of Horses (Equus caballus) in Obollo-Afor Southeastern Nigeria
Strongyliasis Occurs in Epidemic Proportion amongst other Nematodiasis and Cestodiasis of Horses (Equus caballus) in Obollo-Afor Southeastern Nigeria
Uzochukwu Ephraim Emeto, Chukwuemeka Calistus Okolo* and Nwakaego Ernestina Nweze
Department of Veterinary Medicine, University of Nigeria, Nigeria.
Abstract | Nematodiasis and cestodiasis perpetrate serious health and welfare challenges on horses. In countries such as Nigeria, where control of these endoparasites relies on periodic use of anthelminthics, epidemiological evaluations are necessary for deploying meaningful intervention strategies. Such investigations are lacking in the region; hence, the occurrence, risk-factors and nature of cestodiasis and nematodiasis of horses at Obollo-Afor southeastern Nigeria were investigated. Horses billed for slaughter were randomly selected (N = 304) for sampling. About 5grams of faeces and 2ml of blood were collected from each horse into labeled containers for faecal floatation test and haematocrit determination. Gender and body condition scores (BCS) of horses were noted, while age groups and body weight were estimated by dentition and normogram methods respectively. The results showed that 89.9% [confidence interval (CI) 86.4%, 93.4%] of the horses harboured gastrointestinal endoparasites including strongyles (81.3%), Parascaris equorum (8.2%), Oxyuris equi (21.1%), Strongyloides westeri (13.5%), Anoplocephala spp. (10.2%) Paranoplocephala mamillana (3.6%). Polyparasitism was prevalent (43.4%, CI 37.8%, 49.0%). Associations existing between age groups or gender, and the presence of endoparasites were only random. Presence of endoparasite and BCS showed significant (p < 0.05) associations. Horses infected with endoparasites had similar haematocrit values but significantly lower mean body weights when compared to uninfected horses. The study gives the identities and prevalence rates of nematodes and cestodes affecting the horse population. Strongyliasis occured in epidemic proportion within the horse population in the area. This underscores the need for review of extant treatment and control programs.
Editor | Muhammad Abubakar, National Veterinary Laboratories, Park Road, Islamabad, Pakistan.
Received | May 17, 2021; Accepted | April 11, 2022; Published | May 18, 2022
*Correspondence | Chukwuemeka Calistus Okolo, Department of Veterinary Medicine, University of Nigeria, Nigeria; Email: chukwuemeka.okolo@unn.edu.ng, okolomk@gmail.com
Citation | Emeto, U.E., C.C. Okolo and N.E. Nweze. 2022. Strongyliasis occurs in epidemic proportion amongst other nematodiasis and cestodiasis of horses (Equus caballus) in Obollo-Afor southeastern Nigeria. Veterinary Sciences: Research and Reviews, 8(1): 15-22.
DOI | https://dx.doi.org/10.17582/journal.vsrr/2022.8.1.15.22
Keywords | Horse, Nigeria, Nematodes, Cestodes, Strongyliasis
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Gastrointestinal endoparasitoses pose exacting health and welfare challenges to horses, and unfortunately, this group of diseases is sometimes neglected. The deleterious effects of nematodiasis and cestodiasis on equine hosts are enormous, ranging from minor discomfort and reduced productivity to death in some cases (Taylor et al., 2016). In tropical regions of the world, including southeastern Nigeria, the situation is further complicated by prevalence of concurrent vector-borne haemoparasitic diseases (Radostits et al., 2007; Okolo et al., 2020a, b; Emeto et al., 2020); hence, severe illnesses of equine patients are not uncommon. Only a few flukes are considered pathogenic to horses, and they are most often diagnosed at post mortem (Raftery et al., 2017). Horses play host to several round and flat worms such as the large and small strongyles, Strongyloides spp., Oxyuris equi, Parascaris equorum, Anaplocephala spp., Dictyocaulus arnfieldi, and Gastrodiscus aegyptiacus (Atawalna et al., 2015; Saeed et al., 2019). Concurrent infection by more than one parasite-polyparasitism is the rule not the exception in this species (Taylor et al., 2016; Saeed et al., 2019). Colic, afebrile diarrheoa in uncomplicated cases, constipation, inability to thrive, malnutrition, poor productivity, pain, discomfort, morbidity, loose and lusterless hair coat are common signs of gastrointestinal endoparasitosis of horses (Mair et al., 2016). Depending on the organ-system affected more specific signs may be seen; for instance, infestation by haematophagous endoparasites such as Strongylus vulgaris can precipitate blood-loss anaemia, colic, weakness and emaciation, while cough and dyspnoea may dominate the clinical picture in lungworm infections of all age groups of horses or young horses suffering visceral larval migration of Parascaris equorum (Soulsby, 1982; Mair et al., 2016).
The finest control of equine endoparasitism relies on use of integrated helminth control system including interval based approach, evidence based approach, reduction of environmental contamination, grazing management, maintenance of a good balance of the host-parasite equilibrium, and regular review of the control system (Kaplan and Nielsen, 2010; Mathews, 2014). Such integrated system is poorly adopted in Nigeria, and control of equine endoparasitism, to a large extent, relies solely on periodic use of anthelminthics. Epidemiological information on important and common endoparasites of horses in southeastern Nigeria are needed but are wanting. Such pieces of information, when evaluated at regular intervals, are invaluable for design, implementation and review of successful intervention strategies against endoparasitism of horses. Our main aims, therefore, were to determine the common species implicated in nematodiasis and cestodiasis of horses in the area, to determine their level of occurrence, to determine the effect of the infections on the body weights and haematocrits of horses, and to evaluate the significance of risk factors such as age, body condition scores, and gender on the occurrence of the parasites.
Materials and Methods
Study location
The sampling location for the study was the Obollo-Afor horse market. The Obollo-Afor horse market represents the largest aggregation of equids within Enugu state and southeastern Nigeria. It is located within the geographical coordinates 6.9153o N and 7.5139oE (Emeto et al., 2020) within Udenu Local Government Area of Enugu State, Southeastern Nigeria. Further processing of samples after collections were carried out at the research laboratory of Department of Veterinary Medicine, University of Nigeria Nsukka.
Ethical considerations
Ethical considerations for the study were based on procedures of the Animal Use and Care Committee of Faculty of Veterinary Medicine University of Nigeria, which agree with the national institute of health (NIH).
Determination of sample size and sampling procedure
The sample size was determined using the Thrusfield model (Thrushfield, 2007) given below:
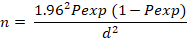
Where; n= required sample size; Pexp= Expected prevalence; d= desired absolute precision.
The following values were set for the parameters: confidence interval= 95%; absolute precision= 5%, expected prevalence= 23.5% as reported by Ehizibolo et al. (2012) on prevalence of horse parasites in northern Nigeria.
Horses billed for slaughter were randomly sampled between February to September 2019. Simple random sampling method was used to select a total of three hundred and four (304) for sampling. For each day of sampling, numbers were assigned to all of the horses billed for slaughter. Random numbers generated using the ‘RANDBETWEEN’ function of Microsoft Excel Software (Microsoft Corporation, USA) in a non-repetitive manner, were utilized for making random selection of 15 horses for sampling on each day. Approximately 5 grams of faeces taken from the rectum of selected animals were collected into labeled faecal sample bottles. Furthermore, about 2mls of blood per subject was collected via jugular venipuncture into labeled sodium-EDTA-treated containers for determination of haematocrit levels. Within one hour of collection, samples were transported to the research laboratory of the Department of Veterinary Medicine for analysis.
Weight and age estimation
For each horse, the heart girth and body length were determined by means of a measuring tape for use in estimation of the body weight by normogram method (Carrol and Huntington, 1988). In addition, body condition scores (BCS) were estimated using standard scoring system (Carrol and Huntington, 1988), where 0 represents very poor, 1 poor, 2 moderate, 3 good, 4 fat, and 5 very fat. The selected horses were grouped into age groups based on physical examination of the dentition as described in (Jeffrey, 1996). Furthermore, the gender of the horses were observed and noted.
Analysis of faecal samples and determination of haematocrits
Faecal samples were analysed using floatation technique as described in Taylor et al. (2016). Briefly, for each sample, approximately 4 grams of faeces was transferred into a plastic container. To it was added about 60 ml of flotation fluid, and the mix was stirred thoroughly. The floatation fluid used was supersaturated sodium chloride solution. Specific gravity of the floatation fluid was measured daily by means of a laboratory hydrometer to ensure that it lies within 1.10–1.20. The faecal suspension was poured through a single layer of cotton gauze into another plastic container. The retained faecal debris was discarded, and immediately the filtered faecal suspension was poured into two vertically standing test tubes till convex menisci were formed at the top. Cover-slips were placed over the test tubes to allow the helminth eggs to float and accumulate underneath them. After about 15 minutes waiting time, the cover-slips were gently transferred onto clean microscope slides for examination under X40 objective lens using light microscopes. Parasite eggs were identified based on their microscopic morphology using atlas of helminth eggs annotated in Soulsby (1982) and Taylor et al. (2016).
The haematocrits of the horses were determined using the micro-haematocrit method (Thrall and Weiser 2002). The haematocrits were read with haematocrit reader and expressed in percentages.
Data analysis and interpretation
Data generated from the cross-sectional study were analysed using descriptive statistics, and results were presented as proportions and percentage with their 95% confidence interval. Chi square and Fisher’s Exact tests were used to check for association between risk factors and the presence or absence of endoparasite infection in horses. The Student’s t-test was used to analyse variations in mean haematocrit values and mean body weights between infected and non-infected horses. Significant associations or differences were accepted at p < 0.05.
Results and Discussion
Following parasitological screening of the trade horses (N= 304), a total of 273 horses (89.9%; 95% confidence interval [CI]: 86.4 - 93.4 %) were positive for gastrointestinal helminthes infections (nematodes and cestodes), 132 horses (43.4%; 95% CI: 37.8 – 49.0 %) were infected with more than one helminth species concurrently (Figure 1). The endoparasite eggs detected and their prevalence were as follows: Strongyle infections (81.3%), Parascaris equorum (8.2%), Oxyuris equi (21.1%), Strongyloides westeri (13.5%), Anoplocephala spp. (10.2%), Paranoplocephala mamillana (3.6%) (Table 1).
In terms of age, 87.5% of horses aged between 5-7 years old were positive for gastrointestinal endoparasites; 198 horses (90.4%) were positive for either gastrointestinal cestodiasis or nematodiasis out of 219 horses within the age bracket of 8-11 years old; while 68 out of 77 horses (83 %) aged ≥ 12 years old were positive to same (Table 2). About 91% of male horses (n = 149) were positive for either gastrointestinal cestodiasis or nematodiasis, while 89% of female horses (n =155) were positive for same (Table 2). Approximately 36%, 86.1% and 96.7%) of horses suffering one form of gastrointestinal cestodiasis or nematodiasis had good (n = 22), moderate (n = 72), and poor (n = 210) body condition scores respectively (Table 2).
In all horses infected with gastrointestinal cestodes or nematodes, associations with gender (X2 = 0.069; p = 0.792) or age group (Fisher’s exact = 0.742; p = 0.665) were only random; however, the association between body condition score and presence of gastrointestinal endoparasites was statistically significant (X2 80.482; df =2; p < 0.01) (Table 2).
There was no statistically significant difference [t (df 302)= 2.39; p= 0.194] between mean haematocrit values of horses with gastrointestinal cestode or nematode infections and those that were uninfected (Table 3). The mean body weight of infected horses were significantly lower [t (df 85.91)= 24.31; p< 0.01] when compared to that of the uninfected group (Table 3).
Table 1: Prevalence of endoparasites identified in horses at the Obollo-Afor horse market.
|
Total number of horses sampled |
304 |
Confidence interval (95%) Lower limit-Upper limit |
|
Gastrointestinal nematodes |
||
|
Strongyle infections |
247 (81.3 %) |
76.9-85.6 |
|
Parascaris equorum |
25 (8.2 %) |
5.1-11.3 |
|
Oxyuris equi |
64 (21.1 %) |
16.5-25.6 |
|
Strongyloides westeri |
41 (13.5 %) |
9.6-17.3 |
|
Cestodes |
||
|
Anoplocephala spp. |
31 (10.2 %) |
6.8-13.6 |
|
Paranoplocephala mamillana |
11 (3.6 %) |
1.5-5.7 |
Percentage positives are shown in parentheses ().
Table 2: Distribution and associated risk factors of gastrointestinal endoparasite infection in trade horses at Obollo-Afor.
|
Risk factors |
Number examined |
Number positive |
Prevalence (%) |
X2 value and p value |
|
Sex |
||||
|
Male |
149 |
135 |
90.6 |
X2 (df1) = 0.069; p = 0.792 |
|
Female |
155 |
138 |
89.0 |
|
|
Age groups |
||||
|
5-7 |
8 |
7 |
87.5 |
Fisher’s exact = 0.742; p = 0.665 |
|
8-11 |
219 |
198 |
90.4 |
|
|
12 and above |
77 |
68 |
88.3 |
|
|
Body condition |
||||
|
Good |
22 |
8 |
36.4 |
X2 (df 2) = 80.482; p < 0.01 |
|
Moderate |
72 |
62 |
86.1 |
|
|
Poor |
210 |
203 |
96.7 |
X2= Yate’s continuity corrected chi-square statistic; df = degree of freedom; significance was accepted at p < 0.05.
Table 3: Mean body weight (kg) and haematocrit values (%) of infected and uninfected trade horses screened for endoparasite infections.
|
Parameters |
Infected horses |
Uninfected horses |
t – statistic; p value |
|
Mean body weight (Kg) ± standard error of mean |
218.33 ± 2.33* |
305.23 ± 2.71 |
t (df 85.91) = 24.31; p < 0.01 |
|
Mean haematocrit values (%) ± standard error of mean |
40.54 ± 0.52 |
42.68 ± 1.5 |
t (df 302) = 1.302; p = 0.194 |
t = student t-test statistic; df = degree of freedom; * indicates significant difference in mean values between infected and uninfected groups at p < 0.05.
The prevalence of gastrointestinal endoparasite infestations among the horse population sampled was remarkably high, with strongyliasis being the singular most common component. In fact, it was shown that nearly nine (9) in every ten (10) horses at the market harboured one form of gastrointestinal (GIT) endoparasite infestation or the other. Mixed endoparasite infections were prevalent (43.4%, N= 304) in the horse population examined involving strongyle infections, Parascaris equorum, Oxyuris equi, Strongyloides westeri, Anoplocephala spp. and Paranoplocephala mamillana. The high prevalence (89.9% N = 304) of GIT endoparasites reported in this study agrees with several reports by previous workers. Useh et al. (2005) in a retrospective study on common diseases found in horses presented at a veterinary teaching hospital in Northern Nigeria over a period of twenty eight years, showed that equine parasitism constituted 82.3% of the total number of cases presented. Furthermore, Wosu and Udobi (2014) reported a prevalence rate of 76.1% (n = 159) for gastrointestinal parasite of horses within the savannah zone of northern Nigeria. In contrast, the prevalence rate from our study is nearly four times higher than that reported by Ehizibolo et al. (2012) who published a prevalence rate of 23.5% for parasitic diseases of horses in Nigeria. Dissimilarities in prevalence rates reported for equine endoparasitosis may depend on whether the horses sampled were intensively, semi-intensively or extensively managed; the value is also affected by variations in sampling or parasitological techniques employed in the research, the presence or absence of veterinary care, as well as variations in weather and climate. For instance, it has been shown that mean parasite count is significantly higher during hot wet season when compared to the value during dry season (Ismail et al., 2016).
Although longitudinal studies modeling the effect of seasonal variations on the prevalence rates of helminthosis in horses in Nigeria are lacking, it is unlikely that the reported high prevalence rates, as seen in this study, would usually plummet during certain seasons, bearing in mind the fact that although the sampling in this study covered the period of peak rainfall (July-September) in southeastern Nigeria, similar studies covering periods of dry season in Nigeria have also reported high prevalence rates as well (Useh et al., 2005; Ishaku et al., 2019).
Infections with strongyle-type parasites dominated the verminous picture from the current study. In fact, about eight in every ten horses sampled haboured one form of strongyle infection or the other. Strongyles are a ubiquitous group of nematodes broadly classified into large and small strongyles: The large strongyles includes Strongylus vulgaris, S. edentatus, S. equinus, and Triodontophorus spp., while the small strongyles (cyathostomins) are made up of over fifty (50) species of strongyle nematodes (Taylor et al., 2016). Before now, large strongyles were considered to be the more harmful parasites of horses; however, in most horse stables, regular worming programs have effectively controlled the problem of large strongyle infections in horses, and small strongyles are now thought to be more important because they are more ubiquitous, they can cause fatal disease, and they have higher tendency to develop anthelminthic resistance (Mathews, 2014). Although small and large strongyle infections cannot be accurately differentiated based on parasite egg morphology (Taylor et al., 2016), it is probable that most of the strongyle eggs detected in this study originated from small strongyle infections. This is so because anaemia is a common feature of large strongyle infections (especially those involving S. vulgaris), but not small strongyle infections (Soulsby, 1982; Mair et al., 2016), in this study, both the infected and uninfected horse groups had similar mean haematocrit values which were within normal ranges suggesting that the infected horses were not anaemic. Furthermore, most anthelmintics in common use within the region have activities mainly against large strongyles, and may have effectively controlled their population.
The potential clinical implication is that horse patients within the region may benefit from special treatments for larval and adult cyathostominosis beyond the routine treatment for other endoparasites, should small strongyle infection be the predominant problem. This would include the use of large doses of febendazole (10mg/kg for 5 consecutive days) or moxidectin which has been more or less reserved for treatment of cyathostominosis (Mathews, 2014). Important auxiliary treatments may include the use of corticosteroids to ameliorate submucosal inflammations in affected horses (Kahn et al., 2010). Several workers reported the preponderance of strongyle infections in equids far and beyond infections by other nematodes from cross-sectional studies (Enigidaw et al., 2015; Jajere et al., 2016; Ishaku et al., 2019). This supports the theory that strongyles are ubiquitous, and extant genetic and environmental factors within and outside the horse favour their lifecycle and survival, perhaps better than what is seen in most other domestic species. In a comparative study, the prevalence of strongyle-type infections in equine, ovine, bovine, and caprine species were reported to be 92.3%, 66.3%, 43.2% and 12.3% respectively, with the rate of infections being highest in equine species (Dogo et al., 2017).
A low prevalence rate (8.2%, N= 304) was recorded for Parascaris equorum infection in the examined horse population. This may be due to the fact that over 90% of horses in the sample population were over 8 years old. It has been established that Parascaris equorum infections and egg shedding are well controlled in immunocompetent adult horses unlike what is seen in foals or immunocompromised horses where the infection is considered to be highly pathogenic (Soulsby, 1982; Taylor et al., 2016). Adult worms of Paranoplocephala mamillana are short-lived and patent infections last for only about 3 months in horses (Taylor et al., 2016); this may account for its very low prevalence rate (3.6% N = 304) as observed in this study.
The results from the study showed that associations between sex or age groups and prevalence of endoparasites were only random, and not significant. This means that either male and female horses or horses belonging to the described age groups (5-7, 8-11, and ≥ 12 years) have equal chances of being infected by any of the identified cestodes or nematodes. The absence of meaningful association between age groups and presence of GIT endoparasite as seen in this study may again be attributed to the fact that more than 90% of horses in the sample population were older horses; therefore, any natural association between young age and higher prevalence of endoparasites may have been successfully distorted by the age distribution of the sample population.
Results from the study showed that nematode and cestode infections were associated with poor body condition score in horses; this was further established by the existence of significant difference between the mean body weight of infected horses (218.33 ± 2.33) and their uninfected counterparts (305.23 ± 2.71, Table 3). This finding fits into the classic theory that parasitism is characterized by inability to thrive, malnourishment, and chronic weight loss in the hosts (Taylor et al., 2016; Mair et al., 2016).
Conclusions and Recommendations
The findings from this study clearly indicate that equine nematode and cestode infections remain a major challenge to equine health within the region, and this calls for review of current control strategies or adoption of new ones by horse care providers. Relying on periodic use of anthelminthics for control of endoparasites is obviously inadequate; therefore, a system that integrates evidence based protocols, good grazing management, reduced environmental contamination among others, is advised.
Acknowledgements
We are grateful to the staff of Veterinary Medicine Laboratory, University of Nigeria for their technical support.
Novelty Statement
Astronomically high prevalence rate of helminthosis of horses at Obollo-Afor was reported. This confirms the need for review of the control approaches currently adopted against helminthosis of horses in the region.
Author’s Contribution
Ephraim Uzochukwu Emeto: Design, collection of data, data analysis and review of manuscript.
Chukwuemekeka Calistus Okolo: Design, collection of data, data analysis, and drafting of manuscript.
Nwakaego Ernestina Nweze: Design, collection of data, and review of manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Atawalna, J., Emikpe, B.O., Sallah, E.K., Shaibu, W., and Folitse, R.O., 2015. The health problems, gastrointestinal and blood parasites commonly associated with donkeys in the upper East region in Ghana. Afr. J. Biomed. Res., 18: 37−41.
Caroll, C.L., and Huntington, P.J., 1998. Body condition scoring and weight estimation of horses. Equine Vet. J., 20(1): 41-45. https://doi.org/10.1111/j.2042-3306.1988.tb01451.x
Dogo, G.I.A., Karaye, P.G., Patrobas, M.G., Galadima, M., and Gosomji, I.J., 2017. Prevalence of gastrointestinal parasites and their impact in domestic animals in Vom Nigeria. Saudi J. Med. Pharmaceut. Sci.m 3: 211-216.
Ehizibolo, D.O., kamani, J., Ehizibolo, P.O., Egwu, K.O., Dogo, G.I. and Salami-Shinaba, J.O., 2012. Prevalence and significance of parasites of horses in some states of northern Nigeria. J. Equine Sci., 23: 1-4. https://doi.org/10.1294/jes.23.1
Emeto, E.U., Okolo, C.C., Nweze, N.E., 2020. Occurrence of Trypannosoma spp. and piroplasm infections of horses at Obollo-Afor southeastern Nigeria and resistance profiles of trypanosomes to isometamidium and diminazene salts. Trop. Anim Health Prod., 52(6): 3745-3753. https://doi.org/10.1007/s11250-020-02412-5
Enigidaw, S., Assefa, A., Mekonnen, N., and Belete, S., 2015. Prevalence of gastrointestinal nematode parasitic infections of horses and donkeys in and around Kombolcha Town. Am-Eurasian J. Sci. Res., 10: 228-234.
Ishaku, B.S., Turdam, B., Abdullahi, M., Anjili, W.I. and Olabode, M., 2019. Endoparasitic infections and the associated risk factors in trade donkeys (Equus Asinus) in Ganawuri district market, Riyom local Government area, plateau state, north central Nigeria. Vet. Sci. Res. Rev., 5: 16-24. https://doi.org/10.17582/journal.vsrr/2019/5.1.16.24
Ismail, A.A., Ahmed, N.K., Bashar, AE., Seri, H.I., Tigani-Asil, T.A., and Abakar, A.D., 2016. A survey of seasonal gastrointestinal parasitic infections in donkeys from a semiarid sub-saharan region Sudan. J. Pathogens. Article ID: 4602751 2016. https://doi.org/10.1155/2016/4602751
Jajere, S.M., Lawal, J.R., Bello, A.M., Wakil, Y., Turaki, U.A., and Waziri, I., 2016. Risk factors associated with the occurrence of gastrointestinal helminths among indigenous donkeys Equus asinus in Northeastern Nigeria. Scientifica, Article ID 3735210. https://doi.org/10.1155/2016/3735210
Jeffrey, D., 1996. Horse dentistry: The theory and practice of equine dental maintenance. Norfolk Printing Company, Nebraska.
Kaplan, R.M. and Nielsen, M.K., 2010. An evidence-based approach to equine parasite control: It ain’t the 60’s anymore. Equine Vet. Educ., 22: 306-316. https://doi.org/10.1111/j.2042-3292.2010.00084.x
Kahn, C.M., Line, S. and Aiello, S.E., 2010. The Merck veterinary manual. Merck and Co. Inc., New Jersey.
Mathews, J.B., 2014. The future of helminth control in horses. Equine Vet. J., 46: 10-11. https://doi.org/10.1111/evj.12200
Mair, T., Love, S., Schumacher, J., Smith, R.K.W. and Frazer, G., 2016. Equine medicine, surgery and reproduction, Saunders, Edinburgh.
Okolo, C.C., Eze, J.I. and Nweze, N.E., 2020a. Hematobiochemical and immunological responses of rats treated with multi-strain probiotics and infected with Trypanosoma brucei. Probiotics and Antimicro. Prot., 12: 952-960. https://doi.org/10.1007/s12602-019-09592-z
Okolo, C.C., Uju, C.N., Chibuzor, D.S., Ezema, K.J., Kolndadacha, O.D., Ibrahim, A., and Aronu, C.J., 2020b. The effects of treatment with multistrain probiotics on serum aminotransferases, AST-ALT ratio, body weight and survivability scores of rats experimentally infected with Trypanosoma brucei. Comp. Clin. Pathol., 29(6): 1229-1236. https://doi.org/10.1007/s00580-020-03175-z
Radostits, O.M., Gay, C.C., Hincnchiff, K.W. and Constaple, P.D., 2007. Veterinary Medicine, a textbook of the disease of cattle, sheep pigs, goats and horses. 10th Edition. W.B. Sounders company, London.
Raftery, A.G., Berman, K.G., and Sutton, D.G.M., 2017. Severe eosinophilic cholangiohepatitis due to fluke infestation in a pony in scotland. Equine Vet. Educ., 29: 196-201. https://doi.org/10.1111/eve.12470
Saeed, M.A., Beveridge, I., Abbas, G., Beasley, A., Bauquier, J., Wilkes, E., Jacobson, C., Hughes, K.J., El-Hage, C., O’Handley, R. and Hurley, J., 2019. Systemic review of gastrointestinal nematodes of horses from Australia. Parasites Vectors, 12: 188. https://doi.org/10.1186/s13071-019-3445-4
Soulsby, E.J.L., 1982. Helminthes, arthropods and protozoa of domesticated animals, Bailliere Tindall, London.
Taylor, M.A., Coop, R.L. and Wall, R.L., 2016. Veterinary Parasitology. Wiley-Blackwell, West Susssex. https://doi.org/10.1002/9781119073680
Thrall, M. A. and Weiser, M.G., 2002. Haematology: Laboratory procedures for veterinary technicians. Mosby Incorporated, Missouri.
Thrusfield, M., 2007. Veterinary epidemiology. Blackwall, USA.
Useh, N.M., Oladele, S.B., Ibrahim, N.D., Nok, A.J., and Esievo, K.A., 2005. Prevalence of Equine diseases in the northern Guinea Savannah of Zaria, Nigeria. J. Equine Sci., 16: 27-28. https://doi.org/10.1294/jes.16.27
Wosu, M.I., and Udobi, S.O., 2014. Prevalence of gastrointestinal helminths of horses equus caballus in the southern guinea savannah zone of Northern Nigeria. J. Vet. Adv., 4: 499-502.
To share on other social networks, click on any share button. What are these?





