Relationship between Body Weight and Morphological Traits in Female and Male Spotted Scat (Scatophagus argus)
Relationship between Body Weight and Morphological Traits in Female and Male Spotted Scat (Scatophagus argus)
Huapu Chen1, Zhiyuan Li1, Yaorong Wang1, Wei Yang2,*, Hongjuan Shi1, Shuisheng Li1,3, Chunhua Zhu1 and Guangli Li1,*
1Fisheries College, Guangdong Ocean University, Guangdong Research Center on Reproductive Control and Breeding Technology of Indigenous Valuable Fish Species, Guangdong Provincial Engineering Laboratory for Mariculture Organism Breeding, Guangdong Provincial Key Laboratory of Pathogenic Biology and Epidemiology for Aquatic Economic Animals, Zhanjiang, 524088, China
2Food and Environmental Engineering Department, Yangjiang Polytechnic, Yangjiang 529566, China
3State Key Laboratory of Biocontrol, and the Guangdong Province Key Laboratory for Aquatic Economic Animals, Sun Yat-Sen University, Guangzhou, China
ABSTRACT
The spotted scat (Scatophagus argus) is an economically important farm fish species found in Southeast China. Production of all-female spotted scat population with superior growth traits can significantly increase farmer’s benefits due to its sexual dimorphism in growth. Herein, the sex ratio and differences in morphometric traits were observed between females and males in a full-sib population of S. argus. Then the correlation between these traits and their effects on body weight (BW) was analyzed. The results showed significant growth differences between males and females. The correlation coefficient between BW and the other 13 traits reached a very significant level, and the strongest correlation was observed in the body height (BH) (male) and body length (BL) (female). In females, BL, body thickness (BT), head length (HL), and BH had a significant and direct effect on BW. The determination coefficients of traits BL, BT, BH, and HL to BW were 0.163, 0.159, 0.057, and 0.018, respectively with the total value being 0.928. In males, the determination coefficients of six traits to BW were 0.124 (BH), 0.192 (BT), 0.053 (BL), 0.018 (OL), 0.0088 (HL), and 0.0092 (PA), with the total value being 0.855. Moreover, two best-fit linear regression equations were constructed in females and males respectively. This knowledge will provide useful and valuable information in better understanding of the differences between both sexes, relationships between morphometric traits, and selective breeding of S.argus. These models might also provide theoretical support for the indirect selection of traits that are difficult to select directly.
Article Information
Received 05 March 2021
Revised 15 April 2021
Accepted 29 April 2021
Available online 30 July 2021
(early access)
Published 18 April 2022
Authors’ Contribution
HC and YW curated the data. ZL, HC and HS perfomed analysis. GL and CZ acquired funding. HC, YW and WY cuducted the research. SL and HS presented the methodology. GL provided the resources. HC and GL wrote the manuscript.
Key words
Scatophagus argus, Sexual dimorphism, Multiple regression equation, Correlation analysis, Path analysis.
DOI: https://dx.doi.org/10.17582/journal.pjz/20210305150335
* Corresponding authors: ligl@gdou.edu.cn;
yangwei516@163.com
0030-9923/2022/0004-1675 $ 9.00/0
Copyright 2022 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Many economic traits in aquatic animals are related to gender. They present obvious dimorphism in growth rate, sexual maturity, and morphology. Growth is one of the most valuable economic traits of fish genetic improvement, and many fish exhibit significant sexual dimorphisms in growth and body size. For example, female individuals of bastard halibut (Paralichthys olivaceus) (Yoneda et al., 2007), rainbow trout (Oncorhynchus mykiss) (Bye and Lincoln, 1986) and European seabass (Dicentrarchus labrax) (Saillant et al., 2001) are larger than male individuals, while yellow catfish (Pelteobagrus fulvidraco) (Wang et al., 2009) and Nile tilapia (Oreochromis niloticus) (Beardmore et al., 2001) show opposite characteristics. Therefore, breeding of full-female or male population possesses greater significance to the hatchery production units. Evaluation of the effects of morphological traits on body weight (BW) using path analysis and multiple regression analysis comprises of the basic study on breeding. The results are used to optimize the breeding strategy, which can improve the economic value of aquatic living (Huo et al., 2010).
In selective breeding, BW is the most direct target trait because of its intuitiveness and measurability (Wang et al., 2016). However, correlation analysis can only measure the degree of association between different traits but the reason for the association is unknown. Therefore, it is necessary to perform path analysis together with correlation analysis (Hardwick and Andrews, 1980; Uckardes et al., 2015). Studies on phenotypic and sexual dimorphism among growth-related traits have been performed in many aquatic species, including Apostichopus japonicaspearl (Zhan et al., 2019), Glossaulax reiniana (Zhao et al., 2014) Pinctada martensii (Deng et al., 2008), Exopalaemon carinicauda (Zhang et al., 2013a) and Patinopecten yessoensis (Chang et al., 2008). In fish species, the main traits affecting the body weight of male tilapia were its full length, depth at caudal peduncle, body width and head length. Whereas the main traits affecting the female body weight were body width, body length, body height and full length (Zeng et al., 2012). In Cynoglossus semilaevis, the body height of females had the highest direct impact on body weight (path coefficient is 0.535), while in males, the total length had the highest direct impact on body weight (Path coefficient is 0.407) (Liu et al., 2015a).
Table I.- Morphological traits of S. argus.
|
Trait |
Male (n=188) |
Female (n=198) |
||
|
Mean±SD1 |
CV2 (%) |
Mean±SD |
CV (%) |
|
|
BW |
74.79±11.57 |
15.47 |
85.60±17.05 |
19.92 |
|
TL |
14.28±0.68 |
4.79 |
14.76±0.94 |
6.39 |
|
BL |
12.11±0.61 |
5.03 |
12.53±0.84 |
6.71 |
|
BH |
6.88±0.35 |
5.21 |
7.22±0.56 |
7.75 |
|
BT |
2.96±0.36 |
12.35 |
3.10±0.34 |
11.12 |
|
PD |
3.59±0.27 |
7.61 |
3.78±0.31 |
8.19 |
|
PV |
3.88±0.31 |
8.22 |
4.08±0.41 |
10.07 |
|
PA |
7.94±0.42 |
5.31 |
8.30±0.57 |
6.95 |
|
HL |
2.95±0.17 |
5.98 |
3.10±0.25 |
8.04 |
|
OLO |
1.58±0.12 |
7.81 |
1.67±0.16 |
9.79 |
|
OL |
0.79±0.05 |
6.69 |
0.82±0.06 |
8.35 |
|
CPL |
1.22±0.15 |
12.69 |
1.27±0.17 |
13.92 |
|
CPH |
1.54±0.08 |
5.49 |
1.60±0.11 |
7.02 |
|
CL |
2.17±0.12 |
5.73 |
2.22±0.15 |
6.79 |
1SD, standard deviation; 2CV, coefficient variation. BW, body weight; TL, total length; BL, body length; BH, depth of body; BT, body thickness; PD, pre-dorsal length; PV, pre-ventral fin length; PA, pre-anal length; HL, head length; OLO, post-orbital length; OL, eye diameter; CPL, caudal peduncle length; CPH, depth at caudal peduncle; CL, caudal length.
Spotted scat (Scatophagus argus) is widely distributed in the mudflats, mangrove swamps, harbors, upstream swamps, estuaries, and marine habitats of Indo-Pacific region, including Southeast China. The adaptation to live in such ever-changing environment endows them with many biological attributes that are highly desirable traits for the cultured finfish (Sivan and Radhakrishnan, 2011; Barry and Fast, 1992). The quality and taste of the fish rank it as an edible fish and the beautifully spotted rhombic body ranks it as a fascinating aquarium fish (Sivan et al., 2007). Previous studies focused on the regulation of growth and reproduction of this fish species (Zhang et al., 2013b; Liu et al., 2015b; Mustapha et al., 2018; Jiang et al., 2019). There is little information about the characteristics of morphological traits available in spotted scat. Although artificial breeding of S. argus has been reported, family breeding is lagging due to the lack of systematic selection breeding research. S. argus usually exhibits significant differences in economically important growth traits between both the sexes. Female individuals generally show a faster growth rate with larger size than males, so fishermen can obtain higher prices in the market (Wang et al., 2018; Deng et al., 2018). Therefore, the objectives of this work were: 1) to determine the association between BW and biometric traits viz. body length (BL), body thickness (BT), head length (HL), depth of body (BH), eye diameter (OL), pre-anal length (PA), pre-ventral fin length (PV), post-orbital length (OLO), caudal peduncle length (CPL), pre-dorsal length (PD), depth at caudal peduncle (CPH) and caudal length (CL) using correlation analysis; 2) to construct a mathematical equation for prediction of BW from biometric traits by multiple regression analysis; and 3) to reveal direct and indirect effects of biometric traits on BW using path analysis. The study will help fishermen for the selection of useful biometric traits during breeding to improve BW.
Materials and methods
Experimental materials
The larvae of S. argus were hatched at Yucheng aquaculture Co., Ltd. in Zhuhai, Guangdong, China. The hatching was carried out in a circular fiberglass tank (0.9 m diameter and 1.0 m height) and then transferred to a cement tank (5.8 m×4.8 m×1.8 m) for cultivation. Spotted scats at one year age were used in this study. A total of 386 individuals (females=198 and males =188) were collected for the experiment. The sex of S. argus was identified based on the gonadal morphology. Animal experiments were conducted in accordance with the relevant guidelines and have been approved by the Animal Research and Ethics Committees of Fisheries College of Guangdong Ocean University (201903004).
Morphometric traits measurement
One day before the experiment, fish were ceased to be fed and anesthetized using MS-222 (Sigma, Saint Louis, MO) with a concentration of 100 mg/L. According to the measurement standard of morphological characteristics reported as the previous report (Gandhi et al., 2013), a total of 14 morphometric traits were measured, including BW, BH, PD, PV, PA, HL, OLO, OL, CPL, CPH, BT, Bl, CL and TL. Electronic balance was employed to measure individual BW for both the sexes and were accurately measured to the 0.01 g resolution, while the other 13 morphological traits measured to the accuracy of 0.01 cm resolution with ImageJ (https://imagej.nih.gov/ij/; accessed February 2021) (Fig. 1). The picture of the fish was obtained from Taiwan Fish Database (https://fishdb.sinica.edu.tw/; accessed February 2021).
Statistical analysis
The statistical analysis of the tested data was done using SPSS statistical package version 20.0 (SPSS Inc., Chicago, IL, USA). Data normality test was carried out using the Shapiro-Wilk test. The coefficient of variation (CV) of each trait was calculated using the following formula:
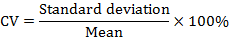
Data for morphometric traits were analyzed using t-tests with statistical significance at P<0.05 and high significance at P<0.01 (Shi et al., 2019).
The path analysis was conducted using SPSS version 20.0 software as described by Du and Chen (2010). Referring to the methods of the Pearson correlation analysis (two-tailed and P-value), the correlation coefficient of each morphological characteristic was divided into direct and indirect effects on BW. The regression relationship between BW and other morphological traits was estimated by first testing the significance of partial regression coefficients of different traits and then by stepwise removing the non-significant morphological traits. The coefficient of determination was calculated by correlation and path analysis, which also determined the coefficient of single or multiple traits to BW. Stepwise multiple regression analysis was used to identify those parameters that significantly contributed to weight traits and optimal multivariate regression equations were established for estimation of BW (Zhao et al., 2014). The below multiple linear regression equation was adopted:
Y = a+b1 X1 +b2 X2 +b3 X3 +b4 X4 +b5 X5 +b6 X6 +b7 X7 +b8 +X8 +b9 X9
Where, Y is dependent variable (BW), a is intercept, b1 - b9 is coefficient of regression, and X1 - X9 is independent variables (biometric traits).
The TL-BW and BH-BW relationship of S. argus was described for each gender using the method potency equation as stated below:
W = aLb
Where, W is BW, L is TL or BH, and a and b are constants.
The coefficient b showed the type of weight increase (isometric, b = 3; positively allometric, b > 3; and negatively allometric, b < 3). The data showed that there was a significant difference when P<0.05 and a high significance was reported when P<0.01. All charts were drawn by means of GraphPad Prism version 8 software and online tools (http://gcloud.taogene.com; accessed February 2021).
Results
Morphometric traits of S. argus
The differences in morphometric traits between female and male S. argus are shown in Figure 2. It was observed that 14 morphometric traits which were measured showed highly significant differences between both the sexes (P<0.01). As a whole, females were significantly bigger than males. In both sexes, the CV values of BW and TL were the highest and lowest among 13 traits, respectively. The CV values of each morphological trait ranged from 4.792% to 15.472% in males and 6.392% to 19.926% in females.
Figure 3 shows the correlation coefficients among the 14 morphological traits of S. argus. Except for BT-PV, BT-OL and CL-OL in females and BW-OL, BT-PV BT-OL and CL-OL in males were not significant, other trait-pairs showed significant positive correlation (P<0.01). All 13 morphometric traits were found to correlate significantly with BW in both the sexes, except for BW-OL in male. The highest correlation coefficient of BW was found in TL (female) and BH (male), with a value of 0.870 and 0.792, respectively.
The direct and indirect effects of the different morphometric traits on BW were evaluated using the path correlation analysis. The morphological traits that showed significant direct effects on BW varied between both the sexes (Table II). In females, four traits (BL, BT, HL, and BH) showed significant direct effects on BW (P<0.05), which ranged from 0.133 (HL) to 0.404 (BL). BL had the largest indirect effect (0.784). In males, six traits (BH, BT, BL, OL, HL, and PA) showed significant direct effects on BW that ranged from -0.133 (OL) to 0.439 (BT). BH had the largest indirect effect (0.926). In both males and females, the indirect effects of all morphometric traits on BW were greater than the direct effects.
The determination coefficients of the morphometric traits on BW are listed in Table III. In the females, the determination coefficient of BL was the largest (0.163), whereas that of the HL was the lowest (0.018). The co-determinant coefficient of BL and BH on BW was found to be the highest, with a value of 0.173. In the males, the determination coefficient of BT was the largest (0.192), whereas that of the OL was the lowest (0.0082). The co-determinant coefficient of BH and BL on BW was found to be the highest, with a value of 0.133. The total determination coefficients in females and males were approximately equal to the multiple correlation coefficients (R2) (Table IV) thus indicating that these traits significantly affect the BW of female and male S. argus.
Table II.- Direct and indirect path coefficients of morphological trait to body weight in female and male S. argus.
|
Gender |
Trait |
CC1 |
DE2 |
IE3 |
||||||
|
BL |
BT |
HL |
BH |
TIE4 |
||||||
|
Female |
BL |
0.868 |
0.404 |
0.173 |
0.247 |
0.365 |
0.784 |
|||
|
BT |
0.702 |
0.399 |
0.171 |
0.070 |
0.180 |
0.421 |
||||
|
HL |
0.578 |
0.133 |
0.081 |
0.023 |
0.071 |
0.176 |
||||
|
BH |
0.854 |
0.238 |
0.215 |
0.107 |
0.128 |
0.450 |
||||
|
CC |
DE |
BH |
BT |
BL |
OL |
HL |
PA |
TIE |
||
|
Male |
BH |
0.792 |
0.353 |
0.142 |
0.290 |
0.093 |
0.167 |
0.234 |
0.926 |
|
|
BT |
0.711 |
0.439 |
0.177 |
0.138 |
-0.049 |
0.074 |
0.126 |
0.466 |
||
|
BL |
0.735 |
0.230 |
0.093 |
0.072 |
0.067 |
0.111 |
0.167 |
0.510 |
||
|
OL |
0.050 |
-0.133 |
-0.054 |
0.015 |
-0.039 |
-0.050 |
-0.051 |
-0.178 |
||
|
HL |
0.447 |
0.094 |
0.038 |
0.016 |
0.046 |
0.035 |
0.051 |
0.185 |
||
|
PA |
0.623 |
0.096 |
0.039 |
0.028 |
0.070 |
0.037 |
0.052 |
0.225 |
||
1CC, correlation coefficient; 2DE, direct effect; 3IE, indirect effect; 4TIE, total of indirect effect. For abbreviations of morphological traits, see Table I.
Table III.- The determination coefficients of morphological traits in male and female fish.
|
Gender |
BL |
BT |
HL |
BH |
TDC1 |
|||
|
Female |
BL |
0.163 |
0.138 |
0.066 |
0.173 |
0.928 |
||
|
BT |
0.159 |
0.019 |
0.102 |
|||||
|
HL |
0.018 |
0.034 |
||||||
|
BH |
0.057 |
|||||||
|
BH |
BT |
BL |
HL |
OL |
PA |
TDC |
||
|
Male |
BH |
0.124 |
0.125 |
0.133 |
-0.025 |
0.031 |
0.045 |
0.855 |
|
BT |
0.192 |
0.064 |
0.013 |
0.014 |
0.024 |
|||
|
BL |
0.053 |
-0.018 |
0.021 |
0.032 |
||||
|
HL |
0.018 |
-0.009 |
-0.010 |
|||||
|
OL |
0.008 |
0.010 |
||||||
|
PV |
0.009 |
|||||||
1TDC, total determination coefficients. Diagonal data are the direct determinant coefficients of single morphological traits on body weight, and above diagonal data are the indirect determinant coefficients of double morphological traits on BW. For abbreviations of morphological traits, see Table I.
The regression relationship between the morphometric traits and BW of female and male S. argus was estimated using a stepwise multiple-regression analysis (Supplementary Table SI). In females, we found that four traits (BL, BT, HL, and BH) showed significant relationships with BW (P < 0.01; Table IV; Supplementary Table SII). A best-fit linear multiple regression equation was constructed as follows:
Y(BW)=-158.521+8.177X1(BL)+19.676X2 (BT) + 9.060X3 (HL)+7.235X4 (BH)
While in males, trait BW showed a significant regression relationship to six traits (BH, BT, BL, HL, OL, PA, and PV) (P < 0.01; Table IV; Supplementary Table SIII). A best-fit linear multiple regression equation was constructed as follows:
Y(BW)=-113.535+11.370X1(BH)+13.863X2(BT)+4.363X3(BL)-28.798X4(OL)+6.140X5(HL)+ 2.631X6(PA)
The constants and coefficients of regression equations were tested by the t-test, which suggested a significant effect on BW (P < 0.05; Table IV; Supplementary Table SIV) in both the sexes.
Table IV.- Analysis of variance (ANOVA) the t-test for constant and partial regression coefficients in multiple regression equation of male and female S. argus.
|
Gender |
Index |
R2 |
Partial coefficient |
SE |
t-value |
p |
|
Female |
Regression analysis |
0.911 |
0.00 |
|||
|
Multiple correlation |
||||||
|
Constant |
-158.52 |
5.84 |
-27.102 |
0.00 |
||
|
BL(X1) |
8.17 |
1.08 |
7.518 |
0.00 |
||
|
BT(X2) |
19.67 |
1.19 |
16.455 |
0.00 |
||
|
HL(X3) |
9.06 |
1.87 |
4.846 |
0.00 |
||
|
BH(X4) |
7.23 |
1.54 |
4.680 |
0.00 |
||
|
Male |
Regression analysis |
0.855 |
||||
|
Multiple correlation |
||||||
|
Constant |
-113.53 |
7.63 |
-14.875 |
0.00 |
||
|
BH(X1) |
11.37 |
1.69 |
6.704 |
0.00 |
||
|
BT(X2) |
13.86 |
1.01 |
13.661 |
0.00 |
||
|
BL(X3) |
4.36 |
1.04 |
4.163 |
0.00 |
||
|
OL(X4) |
-28.79 |
7.05 |
-4.082 |
0.00 |
||
|
HL(X5) |
6.14 |
2.28 |
2.684 |
0.008 |
||
|
PA(X6) |
2.63 |
1.22 |
2.147 |
0.033 |
The TL-BW and BH-BW relationship between female and male S. argus
Analysis of the TL and BW of S. argus in both the sexes showed strong correlations (Fig. 4A). The power regression equations for females and males were y=5.24×10-2X2.74 and y=12.14×10-2X2.41, respectively. The power regression equations of BH and BW in females and males S. argus were y=1.07X2.21 and y=0.81X2.34, respectively (Fig. 4B). The regression coefficient b differed non-significantly from isometric growth (b=3) in both the sexes (P >0.05), thus exhibiting an isometric growth.
Discussion
Even if enhancing production is one of the key objectives in aquaculture, due to the effects of genetic linkage, pleiotropy and environmental factors (Wang et al., 2016; Fu et al., 2015; Toro and Newkirk, 1990), it has been proven difficult to achieve satisfactory results in the selected programs, only taking BW into account. However, BW is highly correlated with many other morphological traits (Pérez-Rostro and Ibarra, 2003). Herein, abundant variation was investigated in the 14 morphological traits of S. argus. The highest CV was found in the BW trait (15.472 and 19.926% in males and females, respectively), while TL was the lowest in males (CV = 4.792%) followed by females (CV = 6.392%). This high variation in growth traits can provide sufficient materials for economic performance selection (Jiang et al., 2014).
Females were significantly bigger than males when considering 14 traits (BW, TL, BL, BH, BT, PD, PV, PA, HL, OLO, OL, CPL, CPH and CL; P < 0.01). This is also in line with the previous research result (Gupta, 2016). This result indicated that females grew significantly faster and bigger than the males in S. argus. Similar phenomena were found in bastard halibut (Paralichthys olivaceus) (Yoneda et al., 2007), rainbow trout (Oncorhynchus mykiss) (Bye and Lincoln, 1986) and European seabass (Dicentrarchus labrax) (Saillant et al., 2001). In contrast, males grew faster and bigger than the females in case of yellow catfish (Pelteobagrus fulvidraco) (Wang et al., 2009) and Nile tilapia (Oreochromis niloticus) (Beardmore et al., 2001).
Correlation analysis indicated that all 13 morphometric traits were significantly correlated with BW in case of both males and females belonging to S. argus (P < 0.01), thus suggesting its feasibility to pick one target trait by selecting other traits in the artificial breeding of S. argus (Ma et al., 2013). Morphological traits are ordinarily employed as a reference for selection in artificial breeding programs (Wang et al., 2016; Liu et al., 2016). In sutchi catfish (Pangasianodon hypophthalmus), full length, head width, body width, and depth at the caudal peduncle are closely related to the BW (An et al., 2013). In pompano (Trachinotus ovatus), full length, body height, body width, and tail stalk height were found to be the main traits affecting the quality of BW (Cheng et al., 2016).
Using only the correlation coefficient between the morphological characteristics and BW may not fully explain all aspects of the relationship within them, and it necessitates investigation of the causes of these relationships (Xiao et al., 2011). The results of path analysis and regression analysis showed that four (BL, BT, HL, and BH) and six (BH, BT, BL, OL, HL, and PA) morphological traits have significant direct effects on BW (P< 0.05) in females and males, respectively. The multiple correlation coefficients between morphological traits and BW were high (more than 0.85) in both females and males, and approximately equal to the determination coefficients. Furthermore, the four and the six morphological traits could be considered as the key factors affecting BW in both female and male of S. argus. According to Wang et al. (2013), the main factor affecting BW is the trait that makes individuals have larger geometric spaces. This conclusion has also been verified in aquatic organisms, such as turbot (Scophthalmus maximus) (Wang et al., 2008), pompano (Trachinotus ovatus) (Ou et al., 2013) and Chinese mitten crab (Eriocheir sinensis) (Geng et al., 2007). This may be due to the large geometric space, which is conducive to the accumulation and storage of fat, protein, and other nutrients. In this study, the direct effect of BT on BW was found to be greater than the indirect effect in female spotted scat suggesting that S. argus may increase the body surface area through BT, thereby increasing the accumulation of nutrients. The reason for this result may be because of the development of the female ovary that results in swollen abdomen.
Conclusions
In this study, we investigated the differences in morphometric traits between females and males, the correlation between 14 morphometric traits, and the direct and indirect effects of morphological traits on BW in S. argus. Using these methods, four and six morphometric traits were identified having significant effects on the BW of females and males. A multiple regression equation was constructed with respect to the BW and morphological traits in females and males separately. This knowledge will provide useful and valuable information in better understanding of the differences between sexes, relationships between growth traits and artificial selective breeding in S. argus, and other closely related species through identification traits related to BW and other important economic traits.
Acknowledgment
This study was supported by grants from National Key R&D Program of China (2018YFD0901203), Special key projects of Guangdong science and Technology Department’s science and technology innovation strategy (2018B030311050), Guangdong Basic and Applied Basic Research Foundation (2019A1515010958 and 2019A1515012042), Zhanjiang city science and technology plan projects (2018A01010 and 2018A02004) and Guangdong South China Sea Key Laboratory of Aquaculture for Aquatic Economic Animals, Guangdong Ocean University (No. KFKT2019ZD07).
There is supplementary material associated with this article. Access the material online at: https://dx.doi.org/10.17582/journal.pjz/20210305150335
Statement of conflict of interest
The authors have declared no conflict of interests.
Reference
An, L., Zhu, Y., Fu, P., Zhang, L., Li, X., Dong, X., Yang, P. and Meng, Q., 2013. Mathematical analysis of effects of morphometric attributes on body weight in sutchi catfish Pangasius sutchi. Chin. J. Fish., 26: 5-9.
Barry, T.P. and Fast, A., 1992. Biology of the spotted scat (Scatophagus argus) in the Philippines. Asian Fish. Sci., 5: 163-179.
Beardmore, J., Mair, G. and Lewis, R., 2001. Monosex male production in finfish as exemplified by tilapia: Applications, problems, and prospects. Aquaculture, 197: 283-301. https://doi.org/10.1016/B978-0-444-50913-0.50015-1
Bye, V.J. and Lincoln, R.F., 1986. Commercial methods for the control of sexual maturation in rainbow trout (Salmo gairdneri R.). Aquaculture, 57: 299-309. https://doi.org/10.1016/0044-8486(86)90208-5
Chang, Y., Zhang, C., Cao, X., Yan, X. and Lin, Y., 2008. Effect of morphometrical traits on weight traits in oneyear old yesso scallop Patinopecten yessoensis. J. Dalian Fish. Univ., 23: 330-334.
Cheng, D., Guo, H., Ma, Z., Jiang, S., Liu, X., Yang, Q. and Li, T., 2016. Mathematical analysis of morphometric attribute effects on body weight for threemonthold Trachinotus ovatus. Mar. Fish., 38: 26-34.
Deng, S.P., Chen, H.P., Zhai, Y., Jia, L.Y., Liu, J.Y., Wang, M. and Li, G.L., 2018. Molecular cloning, characterization and expression analysis of spexin in spotted scat (Scatophagus argus). Gen. Comp. Endocrinol., 266: 60-66. https://doi.org/10.1016/j.ygcen.2018.04.018
Deng, Y.W., Du, X.D., Wang, Q.H., Fu, S. and Huang, R., 2008. Correlation and path analysis for growth traits in F1 population of pearl oyster Pinctada martensii. Mar. Sci. Bull., 10: 68-73.
Du, J. and Chen, Z., 2010. Carrying out path analysis by linear regression of SPSS. B. Biol., 45: 46.
Fu, J., Shen, Y., Xu, X., Liu, C. and Li, J., 2015. Genetic parameter estimates and genotype by environment interaction analyses for early growth traits in grass carp (Ctenopharyngodon idella). Aquacul. Int., 23: 1427-1441. https://doi.org/10.1007/s10499-015-9894-7
Gandhi, V., Venkatesan, V. and Zacharia, P., 2013. Biometry analysis, lengthweight relationship and sexual dimorphism of the spotted scat, Scatophagus argus (Linnaeus, 1766) (Perciformes: Scatophagidae) from Gulf of Mannar, southeast coast of India. J. mar. Biol. Assoc. India, 55: 12-16. https://doi.org/10.6024/jmbai.2013.55.1.01743-02
Geng, X., Wang, X., Sun, J. and Zhang, Y., 2007. Morphometric attributes to body weight for juvenile crab, Eriocheir sinensis. Oceanol. Limnol. Sin., 38: 54.
Gupta, S., 2016. An overview on morphology, biology, and culture of spotted scat Scatophagus argus (Linnaeus 1766). Rev. Fish. Sci. Aquacul., 24: 203-212. https://doi.org/10.1080/23308249.2015.1119800
Hardwick, R. and Andrews, D.J.E., 1980. Genotypic and environmental variation in crop yield. A method of estimating the interdependence of the components of yield. Euphytica, 29: 177-188. https://doi.org/10.1007/BF00037265
Huo, Z.M., Yan, X.W., Zhao, L.Q., Zhang, Y.H., Yang, F. and Zhang, G.F., 2010. Effects of shell morphological traits on the weight traits of Manila clam (Ruditapes philippinarum). Acta Ecol. Sin., 30: 251-256. https://doi.org/10.1016/j.chnaes.2010.08.004
Jiang, D.N., Mustapha, U.F., Shi, H.J., Huang, Y.Q., SiTu, J.X., Wang, M. and Zhu, C.H., 2019. Expression and transcriptional regulation of GSDF in spotted scat (Scatophagus argus). Comp. Biochem. Physiol. B: Biochem. mol. Biol., 233: 35-45. https://doi.org/10.1016/j.cbpb.2019.04.002
Jiang, W., Ma, H., Ma, C., Li, S., Liu, Y., Qiao, Z. and Ma, L., 2014. Characteristics of growth traits and their effects on body weight of G1 individuals in the mud crab (Scylla paramamosain). Genet. mol. Res., 13: 6050-6059. https://doi.org/10.4238/2014.August.7.19
Liu, F., Chen, L. and Lou, B., 2016. Correlation and path coefficient analysis on body weight and morphometric traits of small yellow croaker Pseudosciaena polyactis. Oceanol. Limnol. Sin., 47: 655-662.
Liu, F., Chen, S., Liu, X., Liu, Y., Cui, Z. and Deng, H., 2015a. Correlation and path coefficient analysis for body mass and three morphometric traits in the halfsmooth tongue sole (Cynoglossus semilaevis). Acta Oceanol. Sin., 37: 94-102.
Liu, H., Mu, X., Gui, L., Su, M., Li, H., Zhang, G. and Zhang, J., 2015b. Characterization and gonadal expression of FOXL2 relative to Cyp19a genes in spotted scat Scatophagus argus. Gen. Comp. Endocrinol., 561: 6-14. https://doi.org/10.1016/j.gene.2014.12.060
Ma, H.Y., Ma, C.Y., Ma, L.B., Xu, Z., Feng, N.N. and Qiao, Z.G., 2013. Correlation of growthrelated traits and their effects on body weight of the mud crab (Scylla paramamosain). Genet. mol. Res., 1: 4127-4136.
Mustapha, U.F., Jiang, D.N., Liang, Z.H., Gu, H.T., Yang, W., Chen, H.P. and Zhu, C.H., 2018. Malespecific Dmrt1 is a candidate sex determination gene in spotted scat (Scatophagus argus). Aquaculture, 495: 351-358. https://doi.org/10.1016/j.aquaculture.2018.06.009
Ou, Y., Ji, L., Li, J., Fan, C. and Wang, G., 2013. Correlation analysis of major morphometric traits and body weight of selective group at different month ages of Trachinotus ovatus. J. Fish. China, 37: 961-969. https://doi.org/10.3724/SP.J.1231.2013.38599
Pérez-Rostro, C.I. and Ibarra, A.M., 2003. Quantitative genetic parameter estimates for size and growth rate traits in Pacific white shrimp, Penaeus vannamei (Boone 1931) when reared indoors. Aquacul. Res., 34: 543-553. https://doi.org/10.1046/j.1365-2109.2003.00851.x
Saillant, E., Fostier, A., Menu, B., Haffray, P. and Chatain, B.J.A., 2001. Sexual growth dimorphism in sea bass Dicentrarchus labrax. Aquaculture, 202: 371-387. https://doi.org/10.1016/S0044-8486(01)00786-4
Shi, X., Lu, J., Wu, Q., Waiho, K., Aweya, J.J., Fazhan, H. and Lin, F., 2019. Comparative analysis of growth performance between female and male mud crab Scylla paramamosain crablets: Evidences from a fourmonth successive growth experiment. Aquaculture, 505: 351-362. https://doi.org/10.1016/j.aquaculture.2019.02.062
Sivan, G. and Radhakrishnan, C., 2011. Food, feeding habits and biochemical composition of Scatophagus argus. Turk. J. Fish. aquat. Sci., 11: 603-608.
Sivan, G., Venketesvaran, K. and Radhakrishnan, C., 2007. Biological and biochemical properties of Scatophagus argus venom. Toxicon, 50: 563-571. https://doi.org/10.1016/j.toxicon.2007.05.002
Toro, J.E. and Newkirk, G.F., 1990. Divergent selection for growth rate in the European oyster Ostrea edulis: Response to selection and estimation of genetic parameters. Mar. Ecol. Progr. Ser., 62: 219-227. https://doi.org/10.3354/meps062219
Uckardes, F., Narinc, D. and Kucukonder, H., 2015. Establishment of optimum regression models and determination of relationships between body measurements and slaughter traits in Japanese quails by path analysis. Anim. Prod. Sci., 55: 799-803. https://doi.org/10.1071/AN13357
Wang, D., Mao, H.L., Chen, H.X., Liu, H.Q. and Gui, J.F., 2009. Isolation of Y- and X-linked SCAR markers in yellow catfish and application in the production of all-male populations. Anim. Genet., 40: 978-981. https://doi.org/10.1111/j.1365-2052.2009.01941.x
Wang, M., Deng, S.P., Chen, H.P., Jiang, D.N., Tian, C.X., Yang, W. and Li, G.L., 2018. Phoenixin participated in regulation of food intake and growth in spotted scat, Scatophagus argus. Comp. Biochem. Physiol. B: Biochem. mol. Biol., 226: 36-44. https://doi.org/10.1016/j.cbpb.2018.07.007
Wang, W., Ma, C., Chen, W., Ma, H., Zhang, H., Meng, Y. and Ma, L., 2016. Optimization of selective breeding through analysis of morphological traits in Chinese sea bass (Lateolabrax maculatus). Genet. mol. Res., 15: 1503. https://doi.org/10.4238/gmr.15038285
Wang, X.A., Ma, A.J., Zhuang, Z.M., Li, W.Y., Yue, L., Zou, J. and Wang, T., 2013. Effects of morphometric attributes on body weight of Takifugu rubripes (Temminck Et Schlegel). Oceanol. Limnol. Sin., 44: 135-139.
Wang, X., Ma, A., Xu, K., Lei, J., Yang, Z. and Qu, J., 2008. Relationship between morphometric attributes and body weight of juvenile turbots Scophthalmus maximus. Acta Zool. Sin., 54: 540-545.
Xiao, S., Fu, Z. and Yu, Z., 2011. Path analysis of quantitative traits of male and female Hong Kong oyster Crassostrea hongkongensis. S. China Fish. Sci., 7: 1-9.
Yoneda, M., Kurita, Y., Kitagawa, D., Ito, M., Tomiyama, T., Goto, T. and Takahashi, K., 2007. Age validation and growth variability of Japanese flounder Paralichthys olivaceus off the Pacific coast of northern Japan. Fish. Sci., 73: 585-592. https://doi.org/10.1111/j.1444-2906.2007.01371.x
Zeng, L., Lin, Y., Zhang, Y., Chen, Z., Gan, X., Li, L. and Tang, Z., 2012. Path analysis on morphological traits and body weight of Oreochromis aureus. Southw. China J. agric. Sci., 25: 295-301.
Zhan, Y., Zhang, W., Ge, C., Lin, K., Li, G., Song, J. and Chang, Y., 2019. Relationships between body weight and other morphological traits in young sea cucumbers Apostichopus japonicas. J. Oceanol. Limnol., 37: 759-766.
Zhang, C., Li, F. and Xiang, J., 2013. Path analysis of effects of morphometric attributes on body weight of Exopalaemon carinicauda. J. Fish. China, 37: 809-815. https://doi.org/10.3724/SP.J.1231.2013.38465
Zhang, M.Z., Li, G.L., Zhu, C.H. and Deng, S.P., 2013. Effects of fish oil on ovarian development in spotted scat (Scatophagus argus). Anim. Reprod. Sci., 141: 90-97. https://doi.org/10.1016/j.anireprosci.2013.06.020
Zhao, L., He, Y., Yang, F., Nie, H. and Yan, X., 2014. Correlation and path analysis of morphological and weight traits in marine gastropod Glossaulax reiniana. Chin. J. Oceanol. Limnol., 32: 821-827. https://doi.org/10.1007/s00343-014-3290-4
To share on other social networks, click on any share button. What are these?









