Nematicidal Properties of Some Yeast Culture Filtrates against Meloidogyne javanica Infecting Squash Plants (In Vitro and In Vivo)
Nematicidal Properties of Some Yeast Culture Filtrates against Meloidogyne javanica Infecting Squash Plants (In Vitro and In Vivo)
Atef El-Sagheer1*, Anas El-Mesalamy1, Abdel-Monem Anany2 and Nashaat Mahmoud1
1Agricultural Zoology and Nematology Department, Faculty of Agriculture, Al-Azhar University, Assiut, Egypt; 2Agricultural Zoology and Nematology Department, Faculty of Agriculture, Al-Azhar University, Cairo, Egypt.
Abstract | The influence of some yeasts culture filtrate of Candida oleophila, Cryptococcus albidus, Pichia guilliermondii, Saccharomyces cerevisiae and Sporobolomyces roseus include 103, 106 and 1012 cells, on hatching of second-stage juveniles (J2) of Meloidogyne javanica were estimated in laboratory and greenhouse conditions. Results revealed that all the tested culture filtrates caused significant reduction in number of J2 in all treatments, compared to the non-inoculated control, where the hatching inhibition and mortality percentages increased with an increase in exposure time. At 24 hours, increased the mean number of J2 hatched slowly than in first stages of experiment. Similar patterns showed against M. javanica infecting squash plants under greenhouse conditions. Data indicated that treatment with P. guilliermondii filtrate (1012) caused best percentage of nematode reduction (71.46%). Followed by S. roseus at (1012) (42.95 %), then in third treatment with C. albidus (103) by (40.50 %). On the other hand, all tested treatments decreased the negative effects of nematodes and enhanced growth characters of squash plants. The highest enhancement in the growth characters were recorded in treatment with P. guilliermondii filtrate at (1012).
Received | November 23, 2021; Accepted | December 20, 2021; Published | December 22, 2021
*Correspondence | Atef El-Sagheer, Agricultural Zoology and Nematology Department, Faculty of Agriculture, Al-Azhar University, Assiut, Egypt; Email: atefelsagheer@azhar.edu.eg
Citation | El-Sagheer, A., El-Mesalamy, A., Anany, A-M., and Mahmoud, N., 2021. Nematicidal properties of some yeast culture filtrates against Meloidogyne javanica infecting squash plants (in vitro and in vivo). Pakistan Journal of Nematology, 39(2): 111-121.
DOI | https://dx.doi.org/10.17582/journal.pjn/2021.39.2.111.121
Keywords | Root-knot nematodes, Meloidogyne javanica, Yeasts culture filtrate, Squash, Biocontrol
Introduction
Genus Meloidogyne (Tylenchida: Meloidogynidae) has been of interest to nematologists worldwide probably due to their widespread distribution and success as parasites of economically important crops and is considered as one of the most important genera of plant parasitic nematodes. The root-knot nematodes, Meloidogyne javanica (Treub) Chitwood (1949) the most common pest nematode species in vegetables greenhouses (Koenning et al., 1999), which represented about 10% of total losses caused by plant pests and pathogens combined on vegetables production (Barker and Koenning, 1998). Biological control has become an attractive alternative strategy for the control of plant diseases to reduce the excessive use of agrochemicals and its health hazards, also, multiple beneficial characters such as rhizosphere competence, antagonistic potential. As biocontrol agents many fungi are known to produce nematicidal compounds (Meyer et al., 2000). Many experiments have been made to use antagonistic fungi and compounds produced by these microbes its compounds to reduced phytonematodes and promoting of plant growth (Ashraf and Khan, 2010). Some yeasts are reported to reduce effectively various plant pathogenic of fruits (Fan and Tian, 2001). Potential use of yeast culture products fungi as biocontrol agents of soil-borne plant pathogens and plant growth promoters was recently investigated (Azzam et al., 2012). Recently, a great attention has been given to the application of yeasts to inhibition various plant pathogens (Hashem et al., 2008). The activity of yeast culture components which have nematicidal properties on viability of phytonematodes through inconstancy of rhizosphere ecology and inhibition of hatching process, juvenile development and root-finding (Fernández et al., 2001). Youssef and Soliman (1997) found that the Egyptian isolate of Saccharomyces cerevisiae reduced Meloidogyne incognita populations and improved the growth on Hyoscyamus muticus, also, the application of Saccharomyces cerevisiae as a biocontrol agent of the root-knot nematode, reducing the nematode reproduction ability on cucumber under greenhouse and field conditions (Karajeh, 2013). So this study aims to evaluate some yeasts culture filtrate as biocontrol tools for their application in filed conditions.
Materials and Methods
Purification yeasts culture filtrate
Five tested yeasts culture filtrate of Candida oleophila, Cryptococcus albidus, Pichia guilliermondii, Saccharomyces cerevisiae and Sporobolomyces roseus were grown on the medium using shaking incubator (200 rpm) for 3 days at 30° C then, the cells were harvested by centrifugation at 10000 rpm for 20 min, the culture medium was discarded. The supernatant was filtered by passing the culture broth through a sterile membrane filter 0.2 μm according to the method described by El-Boghdady (1993).
In vitro assay
Effect of culture filtrates on egg-masses and eggs hatching
In vitro test ten egg-masses in uniform in medium size, age and undifferentiated, immersed in 5 ml of three culture filtrate that contains three concentrations of yeasts strains at 103, 106 and 1012 cells prepared in sterile distilled water, egg-masses in plain water in cup supplied with nematode suspension was used as a control. Each treatment was replicated three replications. Cups were loosely covered which slightly permit aeration and reduce evaporation, at 30 ± 5°C. The number of second-stage juveniles hatched was estimated using nematode counting slide in accordance with the Hussey and Barker (1973) technique modified by (Boneti and Ferraz (1981), using an inverted microscope at 40x. The number of second-stage juveniles observed at 6, 24, 48, 72, 96, 168 and 240 hours. Egg-masses hatching inhibition percentage and mean hatching per egg-mass was calculated using the following formula:
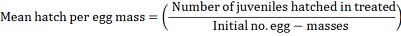

Effect of yeasts culture filtrate on juveniles mortality
For the extraction of Meloidogyne egg-masses and J2 the method in Hussey and Barker (1973) was used. Egg-masses were handpicked from the galled tomato roots from stock cultures, and incubated in sterile distilled water at room conditions at 30±5°C for 48 hours. Hatched second stage juveniles (J2s) that had passed through the tissue paper into the petri dish were counted and concentrated until reached contented 1 ml of distilled water approximately 150 second stage juveniles used each for all the treatments including the control, population density of second stage juveniles in stock suspension (150 J2s/1ml dw) was considered mean population number from 3 times of one ml of stock suspension. 1ml of this juveniles suspension poured in screw-capped test tubes which contained 5 ml of different concentrations of yeasts strains filtrate timely preparation and incubated at 30±5°C for four days and the numbers of dead juveniles were counted at 12,24,48 and 96 hours. Juveniles were considered dead if they did not move when teased with fine needle and body become straight (Cayrol et al., 1989). Each treatment was replicated three replications, distilled water served as control. The mortality percentage was calculated according to the Abbott’s formula.


In vivo assay
Squash seeds cv. Mabrouka® (Hy 42095) (registration no. 1508 – 1998 in ministry of agriculture and land reclamation by Hytech seed company) were sown in pots (20 cm dia.) sterilized clay filled with mixture of clay: sand (1: 3, v: v). After germination, plants were thinned to one per pot and treated with 3000 freshly J2/ pot, poured into five holes in the soil around the plant. Pots were treated with highest effecting rate resulted from invitro assay of culture filtrate which were C. oleophila (103), C.albidus (106), P. guilliermondii (1012), S. cerevisiae (106) and S. roseus (1012), as a soil drench after nematode inoculation. Treatments were replicated three times. The pots were arranged in a randomized complete block design, maintained at 32 ±5 °C and watered as needed. The experiment was terminated six weeks after nematode inoculation. Numbers of galls, egg-masses, developmental stages number, adult female and number of eggs/egg mass were determined, processed for nematode extraction according to methods described by Christie and Perry (1951) and Southey (1986). Nematode final population (Pf) calculated by the following formula:
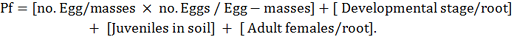
The reproduction rate was calculated by dividing the final population (Pf) by the initial one (Pi). The percentage of nematode reduction calculated by the following formula:
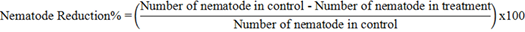
Also fresh and weights of the root and shoot systems as well as their lengths were determined.
The percentage reduction/ increase in growth parameters were calculated by the following formula:
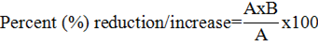
Where; A= value of control, B= value of treatment.
Statistical analyses
The experiment designed with randomized complete design. All the data were subjected to Analysis of Variance (ANOVA) using Costat package version 6.311. The means were compared according to Duncan’s multiple range tests at P ≤0.05 (Duncan, 1955).
Results and Discussion
Effect of some yeasts culture filtrate on hatching of M. javanica in vitro
The influence of some yeasts fungi culture filtrate of Candida oleophila, Cryptococcus albidus, Pichia guilliermondii, Saccharomyces cerevisiae and Sporobolomyces roseus at concs: 103, 106 and 1012 cells of each, on hatching of egg-masses of Meloidogyne javanica were estimated in laboratory conditions. Data presented in (Tables 1 and 2) revealed that, all the tested culture filtrates caused significantly reduction (P≤0.05) in number of second-stage juveniles (J2) hatched from egg-masses and hatching inhibition percentage at all treatments, compared to the non-inoculated control. Data showed that, in all treatments after six hours of exposure no second-stage juveniles hatching where observed which means that all the treatments have no effect on the egg-masses as compared with control (distilled water only), which achieved mean (1.33) J2S hatched from egg-masses. But with increasing exposure time to 12 hours significant reduction was observed between treatments and control but not significant between treatments, in all concs where in 103 best effect recorded in number of hatched second-stage juveniles associated with C. albidus followed by S. cerevisiae then P. guilliermondii, C. oleophila and S. roseus (0.0, 0.33, 0.0 ,0.0 and 1.0), respectively as compared with control (3.0) in this period while in hatching inhibition percentage best results was achieved in S. cerevisiae with (33.34%) followed by S. roseus (11.0%), While in second diluation of 106 same results was achieved in P. guilliermondii, S. cerevisiae and S. roseus (3.3) in hatched J2S and percentage of hatching inhibition (11.0). Also, in high conc. 1012, C. oleophila exibited best data) in hatched J2S and percentage of hatching inhibition flowed by P. guilliermondii, S. cerevisiae (0.0, 0.33 and 3.3) then C. albidus and S. roseus (1.33 and 1.33) and (0.0,11.0, 11.0, 44.34 and 44.34), respectively as compared with control (3.0). After 48 hours S. cerevisiae recorded best effect in all concs (5.67, 4.0 and 3.67), respectively, followed by C. oleophila (6.67, 8.33 and 4.33) and P. guilliermondii (8.0, 11.33 and 5.0), respectively as compared with control (20.67). After 96 hours, in conc. 103 the best results associated with S. cerevisiae hatched J2S and hatching inhibition % (5.77 and 40.0%) followed by C. oleophila (6.33 and 21.1%) and P. guilliermondii (7.33 and24.44%) and least effect in S. roseus (١٢.0 and19.24%), respectively. In 106 S. cerevisiae showed best results (5.33 and 82.33%) followed by S. roseus (8.33 and 27.77%) and C. albidus (8.33 and 2.77%) as compared with control (30.0).
In the end of test at 240 hours, increased the mean number of JS2 hatched slowly than in first stages of experiment. And with increased in time, the effect was decreased; data recorded significant reduction (P≤0.05) in number of J2 hatched from egg-masses and hatching inhibition percentage at all treatments where, in first conc. (103) C. oleophila recorded highest reduction in hatched J2 and hatching inhibition percentage as reduction inhibition over the control in
Table 2: Evaluation of some yeasts culture filtrate on egg-masses hatching inhibition percentage of Meloidogyne javanica in vitro.
|
Treatments |
Conc. |
* Hatching inhibition % |
*Total hatch |
Mean hatch per egg mass |
||||||||
|
6 Hrs. |
1 2 Hrs. |
24 Hrs. |
48 Hrs. |
72 Hrs. |
96 Hrs. |
120 Hrs. |
١٦٨ Hrs. |
240** Hrs. |
||||
|
C. oleophila |
103 |
- |
0 |
45.44 |
41.95 |
35.29 |
21.1 |
14.81 |
13.11 |
12.68 |
6.00 |
0.60 |
|
106 |
- |
0 |
63.58 |
45.14 |
42.66 |
41.1 |
34.25 |
35.24 |
30.99 |
14.67 |
1.47 |
|
|
1012 |
- |
0 |
29.49 |
25.79 |
29.43 |
30 |
33.33 |
29.51 |
21.83 |
10.33 |
1.03 |
|
|
C. albidus |
103 |
- |
0 |
18.21 |
32.27 |
32.34 |
34.44 |
29.64 |
45.91 |
38.73 |
18.33 |
1.83 |
|
106 |
- |
0 |
45.47 |
40.3 |
39.71 |
27.77 |
23.14 |
18.86 |
15.49 |
7.33 |
0.73 |
|
|
1012 |
- |
44.34 |
29.52 |
20.95 |
20.6 |
18.9 |
26.87 |
23.78 |
20.44 |
9.67 |
0.97 |
|
|
P.guilliermondii |
103 |
- |
0 |
40.9 |
38.71 |
29.43 |
24.44 |
34.25 |
42.62 |
36.62 |
17.33 |
1.73 |
|
106 |
- |
11 |
63.6 |
54.82 |
29.43 |
28.9 |
37.03 |
32.78 |
26.77 |
12.67 |
1.27 |
|
|
1012 |
- |
11 |
18.21 |
24.19 |
14.69 |
5.57 |
24.09 |
18.86 |
17.6 |
8.33 |
0.83 |
|
|
S. cerevisiae |
103 |
- |
33.34 |
49.97 |
61.3 |
54.39 |
40 |
40.75 |
36.89 |
30.28 |
14.33 |
1.43 |
|
106 |
- |
11 |
29.52 |
20.95 |
14.69 |
82.33 |
14.81 |
11.49 |
9.15 |
4.33 |
0.43 |
|
|
1012 |
- |
44.34 |
6.82 |
22.6 |
20.6 |
23.34 |
23.14 |
19.68 |
18.32 |
8.67 |
0.87 |
|
|
S. roseus |
103 |
- |
11 |
31.84 |
27.44 |
25.02 |
19.24 |
17.23 |
45.91 |
56.35 |
26.67 |
2.67 |
|
106 |
- |
11 |
27.24 |
19.36 |
25.02 |
27.77 |
23.14 |
29.51 |
26.06 |
12.33 |
1.23 |
|
|
1012 |
- |
11 |
27.24 |
17.76 |
14.69 |
5.57 |
15.75 |
15.57 |
15.49 |
7.33 |
0.73 |
|
*, Increasing inhibition over the control in percentage; **, Total hatch represented mean number of cumulative juveniles hatched after 240. Hrs.- Initial number of egg-masses = 10 egg-masses; uniform size, age and undifferentiated.
percentage (6.0 and 12.68%) as compared with other treatments; S. cerevisiae (14.33 and ٣٠.٢٨%), P. guilliermondii (17.33 and ٣٦.٦٢%), C. albidus (١٨.٣٣ and ٣٨.٧٣%) and S. roseus (26.67 and 56.35%) and control (47.33). While in second conc. data recorded showed highest reduction in S. cerevisiae, followed by C. albidus, S. roseus, P. guilliermondii and C. oleophila. While the highest conc. showed no any significant reduction (P≤0.05) in number of second-stage juveniles hatched from egg-masses and hatching inhibition percentage in all treatments, results ranged from (7.33 and 21.83%) in Sporobolomyces roseus to 10.33 and 15.49 % in C. oleophila compared with control (47.33). Finally, in the end of test data showed that using of yeasts culture filtrate in high conc. 1012 showed significantly reduction (P≤0.05) in mean number of second-stage juveniles hatched from egg-masses and hatching inhibition compared with non-treated control.
Evaluation of some yeasts culture filtrate on the mobility and mortality of M. javanica in vitro
The effect of previous yeasts culture filtrate of each on mortality of Meloidogyne javanica were evaluated in laboratory conditions. Data presented in (Tables 3 and 4) revealed that, all the tested culture filtrate caused significant differences (P≤0.05) in the dead number of second-stage juveniles and mortality percentages at all treatments, compared to the non-inoculated control. In all treatments and control after six hours of exposure no dead second-stage juveniles were observed. But results showed significant differences in other concentrations. After 12 hours at 103 the best result was observed in C. albidus that equal with P. guilliermondii in dead number of second-stage juveniles and mortality percentages (1.33 and 0.66%) and (1.33 and 0.66%), respectively, and moderate effect was seen in C. oleophila (2.33 and1.68%) followed by S. cerevisiae (2.0 and1.34%), and maximum effect was exhibited by S.roseus (3.0 and 2.34%), respectively as compared with control (0.67). In second conc. 106 data showed significantly differences between treatments and control but not found in between treatments where the means ranged between (2.0 and1.34%) in C. oleophila shared by P. guilliermondii also followed by (3.33 and 2.68%) in S. roseus. In third conc. 1012 no significant difference was observed between the treatments that differed from the previous conc. Generally, the mortality rates of juveniles increased with an increase in exposure time. Where, increased the exposure time to 24 hours, no difference was observed in the three concs, where in first conc. C. albidus recorded (4.67 and 0.35%) and in second conc. (5.33 and 1.04%), while in third conc. S.roseus recorded (5.67 and 1.40%) as best results. After 48 hours culture filtrate of C. albidus proved most effect with (5.33 and 1.38%) and in second conc. 106 the results remain close to the low conc. Where S. roseus (3.67 and 0.34%) and in highest conc. 1012 C. oleophila with (8.33 and 4.51%), respectively as compared with control in this period (4.00).
After 72 hours effects were evident significantly between the treatments and the control, where P. guilliermondii achieved best effect on dead number of second-stage juveniles and mortality percentages by (11.33 and 6.66%) flowed by S. roseus (8.33 and 3.50%) and in last order C. albidus (5.67 and 0.7%). In the end of test at four days (96 h) in low conc. P. guilliermondii achieved more significant differences effect with (13.0 and 7.77%) flowed by S. roseus (8.33 and 2.81%), in second conc. C. albidus achieved (9.33 and 3.87%) as best data followed by C. oleophila with (7.67 and 2.12%), while in high conc. the best recorded data was associated with S. cerevisiae followed by C. albidus (17.33 and 12.36%) and (17.0 and 12.01%), respectively as compared with control in this period (5.67).
Table 4: Evaluation of yeasts filtrates on percentage mortality of the second stage juveniles of Meloidogyne javanica.
|
Treatments |
Conc. |
Mortality (%) |
|||||
|
6 Hrs |
12 Hrs |
24 Hrs |
48 Hrs |
72 Hrs |
96 Hrs |
||
|
C. oleophila |
103 |
- |
1.68 |
0.70 |
2.09 |
1.05 |
1.75 |
|
106 |
- |
1.34 |
0.70 |
2.09 |
1.40 |
2.12 |
|
|
1012 |
- |
0.66 |
3.13 |
4.51 |
7.02 |
10.24 |
|
|
C. albidus |
103 |
- |
0.66 |
0.35 |
1.38 |
0.70 |
1.75 |
|
106 |
- |
0.66 |
1.04 |
2.09 |
3.50 |
3.87 |
|
|
1012 |
- |
0.66 |
3.83 |
9.37 |
11.23 |
12.01 |
|
|
S. cerevisiae |
103 |
- |
1.34 |
0.35 |
3.12 |
2.81 |
1.06 |
|
106 |
- |
1.34 |
0.35 |
2.09 |
1.75 |
0 |
|
|
1012 |
- |
1.34 |
3.83 |
8.33 |
10.17 |
12.36 |
|
|
S. roseus |
103 |
- |
2.34 |
0.35 |
2.42 |
3.50 |
2.81 |
|
106 |
- |
2.68 |
0.34 |
0.34 |
0.34 |
0.69 |
|
|
1012 |
- |
1.68 |
1.40 |
5.90 |
5.26 |
5.65 |
|
|
P. guilliermondii |
103 |
- |
0.66 |
2.09 |
4.51 |
6.66 |
7.77 |
|
106 |
- |
1.34 |
1.74 |
3.32 |
1.40 |
2.47 |
|
|
1012 |
- |
0.66 |
3.50 |
4.86 |
7.02 |
6.71 |
|
Initial number of juveniles = 150 second stage juveniles; fresh hatching and undifferentiated.
Greenhouse assay
Measurement of Meloidogyne javanica criteria
The influence of previous yeasts culture filtrates against M. javanica infecting squash plants were evaluated under greenhouse conditions. Data presented in Table 5 revealed that, all the tested treatments caused significant reduction (P≤0.05) in the nematode criteria; numbers of root galls, developmental stages, nematodes in soil, number of egg-mass, number of eggs per egg-mass, final population, rate of nematode reproduction and percentages of nematode reduction as compared with control. Where data indicated that treatment with P. guilliermondii filtrate at (1012) caused the best effect on the calculated nematode criteria, rate of nematode reproduction and reduction percentages (2.51 and71.46%), respectively as compared with non-treated control (8.79), respectively, except the number of juveniles in soil (226) where that effect not noted compared to control which recorded (324). Followed by treatment with S. roseus at (1012) which showed (5.01 and 42.95 %), then in third treatment with C. albidus at (103) by (5.23 and40.50 %) in same previous criteria with noting obvious significant effect on the number of juveniles in soil (175) as compared to other treatments and control. And in last ranked treatment with C. oleophila at (103) which caused (5.42 and 38.33 %) and S. cerevisiae at (106) which caused (5.83 and 33.70%).
Measurement of plant growth
One the other hand, all tested treatments decreased the negative effect of nematodes and an enhancement in the growth characters of squash plants, but the effect varied through all tested culture filtrates. Although the effects varied depending on examined concentrations, generally represent a positive effect to reduce the nematodes that were able to penetrate the plants. Data presented in Table 6 reported that the highest increase percentage in shoot and root length and weight were recorded in treatment with P. guilliermondii filtrate at (1012) which caused (37.06% and 50.23%) and highest root length and weight (85.40 and 24.57%) as compared to control treatment followed by treatment with S. roseus at (106) in shoot length and weight (34.71% and 38.12%) and root length and weight (82.59 and 20.53%) (Table 6).
The results obtained in this study showed the use of some yeasts fungi culture filtrate: C. oleophila, C. albidus, P. guilliermondii, S. cerevisiae and Sporobolomyces roseus caused significant reduction on the root-knot nematode M. javanica, this was explained by the reduced hatching and mortality juveniles in vitro. Similar results were obtained by Shawky et al. (2006) who reported that all the bioagent candidates S. uvarum and S. ludwigii proved harmful to M. javanica juveniles, egg-masses, but the effect differed from one candidate to another. As general, the lethal action of toxic compounds produced by microorganisms on egg in vitro noted by Meadows et al. (1989). Data showed that, after six hours of exposure no second-stage juveniles hatching were observed which means that all the treatments have no effect on the egg-masses as compared with control (distilled water only), which achieved mean (1.33) J2S hatched from egg-masses, but with increased exposure time to 12 hours significant reduction was observed between treatments and control but no significant reduction between treatments. And with increased exposure time to 24 hours data began to increase showed significantly reduction (P≤0.05) in means number of second-stage juveniles hatched from egg-masses and hatching inhibition percentage in all treatments as compared with control. Similar results were obtained by Mohamed et al. (2008) indicated that the application with the yeast isolates P. guilliermondii and C. albicans treatments significantly reduced the number of juveniles in vitro after both 24h and ٤٨h. This study showed that by using culture filtrate of P. guilliermondii as bio-agents to root-knot nematode, as reported by Jijakli and Lepoivre (1998) who noted that P. guilliermondii was found to show high effect which may be refer to the activity of β-1,3-glucanase enzyme that could result in the degradation of the cell walls (Jijakli et al., 1999; Masih et al., 2001). On the other side, the influence of previous yeasts culture filtrate against root-knot nematodes, M. javanica infecting squash plants caused significant reduction (P≤0.05) in the nematode criteria compared with control, and an enhancement in the growth characters of squash plants, according to Karajeh (2013), who reported that different yeast strains are promising biocontrol agents for different crops against root-knot nematode infection, which reduced nematode reproduction and increased plant growth parameters (Wu, 2015). The increase growth characters in all treatments are partially due to the effect of the tested culture filtrate on the nematode; besides its role in plant nutrition as suggested by Akhtar and Malik (2000). Where data indicated that treatment with P. guilliermondii filtrate caused the best effect on the nematode criteriam, followed by treatment with Sporobolomyces roseus then in third treatment with C. albidus, and in last ranked treatment with C. oleophila and S. cerevisiae. Similar results were obtained by Shawky et al. (2006) who reported that among all the bioagent filtrates, Saccharomyces spp. proved harmful to M. javanica juveniles, egg-masses and numbers of galls but the effect magnitude differed from one filtrate to another and an enhancement in plants growth. Also, Karajeh (2013) reported that the application of S. cerevisiae as soil drench treatment led to an obvious reduction of root galling caused by M. javanica and resulted in reducing the nematode reproduction ability on cucumber under growth room and field conditions and the yeast was more effective at increase in concentration. Karajeh (2014) noted that the effect of the yeast fungus S. cerevisiae on tomato lead to a significant reduction of root galling and root-knot nematode reproduction ability as compared to the untreated control. Applied the filtrate of S. cerevisiae at high dilution (1012) given the best reduction on the root-knot nematode M. javanica as hatching and mortality juveniles in vitro, this agreement with Youssef and El-Nagdi (2012). Application of S. cerevisiae at the highest concentration caused the highest percentage reduction of juveniles and galls followed by the moderate and the lowest concentrations (Ismail, 2014).
Table 5: Evaluation of some yeasts filtrate on root knot nematode Meloidogyne javanica infecting squash plants under greenhouse conditions.
|
Treatments |
Conc. |
Nematode criteria |
||||||||
|
No. of galls/root system |
No. of nematode |
No. of egg masses/ root |
No. of eggs/egg mass |
Final population |
NRR |
Nematode reduction % |
||||
|
In soil (250 g) |
In root |
Egg laying female |
||||||||
|
Candida oleophila |
103 |
22 b |
145 c |
153 a |
123 b |
107 ab |
148 bc |
16257 |
5.42 |
38.33 |
|
Cryptococcus albidus |
106 |
15 c |
175 bc |
120 bc |
94 bc |
95 b |
161 ab |
15684 |
5.23 |
40.50 |
|
Pichia guilliermondii |
1012 |
14 c |
226 bc |
76 c |
66 c |
53 c |
135 c |
7523 |
2.51 |
71.46 |
|
Saccharomyces cerevisiae |
106 |
26 b |
238 b |
163 a |
148 b |
99 b |
171 ab |
17478 |
5.83 |
33.70 |
|
Sporobolomyces roseus |
1012 |
13 c |
197 bc |
90 c |
81 bc |
90 b |
163 ab |
15038 |
5.01 |
42.95 |
|
Control |
- |
76 a |
324 a |
132 b |
163 a |
130 a |
198 a |
26359 |
8.79 |
- |
|
LSD |
- |
7.26 |
9.72 |
8.61 |
3.59 |
3.98 |
7.29 |
- |
- |
- |
Means at each column followed by the same letter are not significantly different at (P≤0.05) according to Duncan multiple range test. NRR: Nematode rate of reproduction = Pf/Pi
Table 6: Plant growth response to some yeasts filtrate against root knot nematode Meloidogyne javanica infecting squash plants under greenhouse conditions.
|
Treatments |
Rate |
Growth characters |
|||||||
|
Shoot |
Root |
||||||||
|
Length (cm) |
Weight(g) |
Length (cm) |
Weight(g) |
||||||
|
Treated |
Increase % |
Treated |
Increase% |
Treated |
Increase % |
Treated |
Increase % |
||
|
C. oleophila |
103 |
40.27** |
18.45 |
24.80ns |
11.22 |
27.90** |
56.75 |
12.63ns |
10.79 |
|
C. albidus |
1012 |
36.83 ns |
8.33 |
30.05* |
34.76 |
22.70** |
27.53 |
12.30 ns |
7.90 |
|
P. guilliermondii |
1012 |
46.60** |
37.06 |
33.50** |
50.23 |
33.00** |
85.40 |
14.20 * |
24.57 |
|
S. cerevisiae |
106 |
38.00ns |
11.77 |
26.40ns |
18.39 |
24.20** |
35.96 |
12.70 ns |
11.41 |
|
S. roseus |
106 |
45.80** |
34.71 |
30.80** |
38.12 |
32.50** |
82.59 |
13.74 * |
20.53 |
|
Control |
- |
34.00 |
- |
22.30 |
- |
17.80 |
- |
11.40 |
- |
=* Significant at 0.05 level of probability; **= Highly significant at 0.05 level of probability; ns= Non significant at 0.05 level of probability.
Generally, several yeasts fungi (commercially available products or natural) have shown significant disease reduction through various mechanisms to reduce pathogen development either directly through antagonism of soil borne pathogens or indirectly by eliciting a plant-mediated resistance response (Suzzi et al., 1995; Singh, 2014; Nourani et al., 2015), where Punja and Utkhede (2003), reported that yeasts produce secondary metabolites and enzymes that demonstrate toxicity against plant parasitic nematodes; these results suggested that enzymes or other active compounds produced by the fungal culture filtrates exhibit activity against specific stages in life cycle of nematode (Shinya et al., 2008; Li et al., 2015; Bogner et al., 2016) and including loline alkaloids, pyrrolopyrazine, and organic acids that may account for activity against some phytoparasitic nematodes (Porter et al., 1994; Bush et al., 1997). Youssef and Soliman (1997) reported that the effect of yeast on M. incognita might be due to the activity of S. cerevisiae to convert carbohydrates to ethyl alcohol and CO2 toxic to nematodes (Mostafa, 2004; Youssef and El-Nagdi, 2012) Linoleic acid was identified as a nematotoxic compound from Arthrobotrys conoides and Arthrobotrys oligospora (Meyer, 1995). The nematicidal properties may be referred to the fact that most microorganisms act against plant parasitic nematodes by means of metabolic byproducts, enzymes and toxins (Chernin and Chet, 2002). The effects of these toxins include the suppression of nematode reproduction, egg hatching and juvenile survival, as well as the direct killing impact on nematode itself (Siddiqui, 2005).
Acknowledgements
The authors would like to thank to Prof. Dr. Abd El-Latif H. Abd El-Latif, Professor of microbial genetics and environmental meta-genome biotechnology, Genetics Department, Faculty of Agriculture, Beni-Suef University, Egypt, for his contribution to the preparation and processing of tested yeast culture filtrates.
Novelty Statement
The current study demonstrated the ability of yeast culture filtrates to ssuppress root-knot nematodes in addition to increasing plant health.
Author’s Contribution
El-Sagheer, A. conceived designed and performed the experiments, analyzed data, wrote, and reviewed the paper. El-Mesalamy, A. reviewed the first draft. Anany, A. designed the experiment and reviewed the final draft. And Mahmoud N. reviewed the final draft.
Conflict of interest
The authors declare that they have no competing interests.
Funding
Self-funding
Data availability
Availability of data and materials not applicable in this study.
Ethics approval of human data or animal tissues
Not applicable in this section.
Consent for publication
Not applicable in this section.
References
Akhtar, M., and Malik, A., 2000. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: A review. Bioresour. Technol., 74(1): 35-47. https://doi.org/10.1016/S0960-8524(99)00154-6
Ashraf, M.S. and Khan, T.A., 2010. Integrated approach for the management of Meloidogynejavanica on eggplant using oil cakes and biocontrol agents. Arch. Phytopathol. Plant Prot., 43(6): 609-614. https://doi.org/10.1080/03235400801972434
Azzam, S., Karam, N., Hameed, K., Goussous, S., Maraqa, A. and Makhadmeh, I., 2012. Investigation of indigenous plant root associated bacteria and yeast isolates for plant growth promoting ability. Jordan J. Agric. Sci., 8: 1-14.
Barker, K.R. and Koenning, S.R., 1998. Developing sustainable systems for nematode management. Ann. Rev. Phytopathol., 36(1): 165-205. https://doi.org/10.1146/annurev.phyto.36.1.165
Bogner, C.W., Kariuki, G.M., Elashry, A., Sichtermann, G., Buch, A.K., Mishra, B., and Schouten, A., 2016. Fungal root endophytes of tomato from Kenya and their nematode biocontrol potential. Mycol. Prog., 15(3); 30. https://doi.org/10.1007/s11557-016-1169-9
Boneti, J. and Ferraz, S., 1981. Modificação do método de Hussey and Barker paraextração de ovos de Meloidogyneexigua de raízes de cafeeiro. Fitopatol. Brasil., 6(3): 553.
Bush, L.P., Wilkinson, H.H. and Schardl, C.L., 1997. Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiol., 114(1): 1. https://doi.org/10.1104/pp.114.1.1
Cayrol, J.-C., Djian, C. and Pijarowski, L., 1989. Study of the nematicidal properties of the culture filtrate of the nematophagous fungus Paecilomyceslilacinus. Rev. Nematol., 12(4): 331-336.
Chernin, L., and Chet, I., 2002. Microbial enzymes in the biocontrol of plant pathogens and pests. Enzymes in the environment: Activity, ecology, and applications, 171-226. https://doi.org/10.1201/9780203904039.ch7
Chitwood, B.G., 1949. Root-knot nematodes. Part 1. A revision of the genus Meloidogyne goeldi, 1887. Proc. Helminthol. Soc. Washington, 16(2): 90-114.
Christie, J.R., and Perry, V.G., 1951. Removing nematodes from soil. Proc. Helminthol. Soc. Washington, 18(2): 106-108.
Duncan, D.B., 1955. Multiple range and multiple F tests. Biometrics, 11(1): 1-42. https://doi.org/10.2307/3001478
El-Boghdady, M.E., 1993. Integrated postharvest diseases management of certain pome fruits [Anna apple].
Fan, Q. and Tian, S., 2001. Postharvest biological control of grey mold and blue mold on apple by Cryptococcus albidus (Saito) Skinner. Postharv. Boil. Technol., 21(3): 341-350. https://doi.org/10.1016/S0925-5214(00)00182-4
Fernández, C., Rodrı́guez-Kábana, R., Warrior, P. and Kloepper, J.W., 2001. Induced soil suppressiveness to a root-knot nematode species by a nematicide. Biol. Contr., 22(2): 103-114. https://doi.org/10.1006/bcon.2001.0961
Hashem, M., Omran, Y.A. and Sallam, N.M., 2008. Efficacy of yeasts in the management of root-knot nematode Meloidogyneincognita, in Flame Seedless grape vines and the consequent effect on the productivity of the vines. Biocontr. Sci. Technol., 18(4): 357-375. https://doi.org/10.1080/09583150801950568
Hussey, R.S. and Barker, K.R., 1973. A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Dis. Rep., 57: 1025-1028.
Ismail, A.E., 2014. Growing Jatrophacurcas and Jatrophagossypiifolia as ainterculture with sunflower for control of Meloidogynejavanica in Egypt. Int. J. Sustain. Agric. Res., 1(2): 39-44.
Jijakli, M.H., and Lepoivre, P., 1998. Characterization of an exo-β-1, 3-glucanase produced by Pichiaanomala strain K, antagonist of Botrytis cinerea on apples. Phytopathology, 88(4): 335-343. https://doi.org/10.1094/PHYTO.1998.88.4.335
Jijakli, M.H., Lepoivre, P., and Grevesse, C., 1999. Yeast species for biocontrol of apple postharvest diseases: an encouraging case of study for practical use. In Biotechnological approaches in biocontrol of plant pathogens. Springer, Boston, MA. pp. 31-49. https://doi.org/10.1007/978-1-4615-4745-7_2
Karajeh, M.R., 2013. Efficacy of Saccharomyces cerevisiae on controlling the root-knot nematode (Meloidogynejavanica) infection and promoting cucumber growth and yield under laboratory and field conditions. Arch. Phytopathol. Plant Prot., 46(20): 2492-2500. https://doi.org/10.1080/03235408.2013.799819
Karajeh, M.R., 2014. Enhancement of tomato growth, yield and resistance to the root-knot nematode (Meloidogynejavanica) after the field application of Saccharomyces cerevisiae. Hell. Plant Prot. J., 7(1): 35-41.
Koenning, S., Overstreet, C., Noling, J., Donald, P., Becker, J. and Fortnum, B., 1999. Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. J. Nematol., 31(4S): 587.
Li, J., Zou, C., Xu, J., Ji, X., Niu, X., Yang, J., and Zhang, K.Q., 2015. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Ann. Rev. Phytopathol., 53: 67-95. https://doi.org/10.1146/annurev-phyto-080614-120336
Masih, E.I., Slezack-Deschaumes, S., Marmaras, I., Barka, E.A., Vernet, G., Charpentier, C., and Paul, B., 2001. Characterisation of the yeast Pichiamembranifaciens and its possible use in the biological control of Botrytis cinerea, causing the grey mould disease of grapevine. FEMS Microbiol. Lett., 202(2): 227-232. https://doi.org/10.1111/j.1574-6968.2001.tb10808.x
Mayer, A., 1995. (Please give reference title). Ph. D. thesis, University of Kaiserslautern, Kaiserslauten.
Meadows, J., Gill, S. and Bone, L.W., 1989. Factors influencing lethality of Bacillus thuringiensiskurstaki toxin for eggs and larvae of Trichostrongy luscolubriformis (Nematoda). J. Parasitol., pp. 191-194. https://doi.org/10.2307/3282765
Meyer, S.L.F., Massoud, S., Chitwood, D. and Roberts, D., 2000. Evaluation of Trichodermavirens and Burkholderiacepacia for antagonistic activity against root-knot nematode, Meloidogyne incognita. Nematology, 2(8): 871-879. https://doi.org/10.1163/156854100750112815
Mohamed, H., Omran, Y.A., and Sallam, N.M., 2008. Efficacy of yeasts in the management of root knot nematode Meloidogyne incognita, in flame seedless grape vines and the consequent effect on the productivity of the vines. Biocontr. Sci. Technol., 18(4): 357-375. https://doi.org/10.1080/09583150801950568
Mostafa, E.A.M., 2004. Effect of spraying with ascorbic acid, vitamin B and active dry yeast on growth, flowering, leaf mineral status, yield and fruit quality of grand nain banana plants. Ann. Agric. Sci. Ain Shams Univ., 49(2): 643-659.
Nourani, S.L., Mohammadi-Goltapeh, E., Safaie, N., JalaliJavaran, M., Pourjam, E., Shams-Bakhsh, M., and JahanshahiAfshar, F., 2015. The effects of Arthrobotrysoligospora and Arthrobotrysconoides culture filtrates on second stage juvenile mortality and egg hatching of Meloidogyne incognita and Meloidogynejavanica. J. Crop Prot., 4: 667-674.
Porter, J.K., Bacon, C.W., and White, J.F., 1994. Chemical constituents of grass endophytes. Biotechnol. Endophytic Fungi Grasses, pp. 103-123. https://doi.org/10.1201/9781351070324-8
Punja, Z.K. and Utkhede, R.S., 2003. Using fungi and yeasts to manage vegetable crop diseases. Trends Biotechnol., 21(9): 400-407. https://doi.org/10.1016/S0167-7799(03)00193-8
Shawky, S., El-Shennawy, R. and Shady, A., 2006. Biological control of Meloidogynejavanicaon tomato plants with isolated bioagent in Egypt. J. Agric. Sci. Mansoura Univ., 37: 6049-6063.
Shinya, R., Aiuchi, D., Kushida, A., Tani, M., Kuramochi, K., and Koike, M., 2008. Effects of fungal culture filtrates of Verticilliumlecanii (Lecanicillium spp.) hybrid strains on Heteroderaglycines eggs and juveniles. J. Invert. Pathol., 97(3): 291-297. https://doi.org/10.1016/j.jip.2007.11.005
Siddiqui, Z.A., 2005. PGPR: Prospective biocontrol agents of plant pathogens. In PGPR: Biocontrol and bio-fertilization, pp. 111-142. https://doi.org/10.1007/1-4020-4152-7_4
Singh, H., 2014. Management of plant pathogens with microorganisms. Proc. Natl. Acad. Sci., 80: 443-454. https://doi.org/10.16943/ptinsa/2014/v80i2/55120
Southey, J.F., 1986. Laboratory methods for work with plant and soil nematodes. London, UK, Her Majesty’s Stationery Office, pp. 202.
Suzzi, G., Romano, P., Ponti, I., and Montuschi, C., 1995. Natural wine yeasts as biocontrol agents. J. Appl. Bacteriol., 78(3): 304-308. https://doi.org/10.1111/j.1365-2672.1995.tb05030.x
Wu, B., 2015. Effects of mixed application of microorganisms on vegetable growth. Dissertation of Soil and Environmental Science, Zhongxing University, pp. 1-86.
Youssef, M. and Soliman, M.M., 1997. Effect of integrated management on Meloidogyne incognita infecting Egyptian henbane, Hyoscyamusmuticus and on subsequent cowpea plant. 1st Scientific Conference of Agricultural Sciences, Fac. Agric., Assiut Univ., Assiut.
Youssef, M.M.A., and El-Nagdi, W., 2012. Effect of biologically active bread yeast on controlling Meloidogyne incognita infesting green bean and on the yield quantity and quality. Selçuk J. Agric. Sci., 25(4): 30-33.
To share on other social networks, click on any share button. What are these?




