Influence of Planting dates, Cutting types and IBA Concentrations on Rooting of Silvery (Leucophyllum frutescens)
Research Article
Influence of Planting dates, Cutting types and IBA Concentrations on Rooting of Silvery (Leucophyllum frutescens)
Nagina Zeb* and Noor ul Amin
Department of Horticulture, Faculty of Crop Production Sciences, The University of Agriculture, Khyber Pakhtunkhwa, Peshawar, Pakistan.
Abstract | The current experiment was executed to study impact of different planting dates during 2016-2017 (July 01, July 16, July 31, August 15 and August 30) and two cutting types i.e. Hard wood and Semi-hard wood cuttings on rooting response of Silvery treated with various concentrations of IBA (control, 2000, 4000, 6000 and 8000 ppm) at Ornamental Nursery, Horticulture Department, The University of Agriculture Peshawar. The trial was arranged in Randomized Complete Block Design (RCBD) with split-split plot arrangement. Planting dates were reserved in main plot, cutting types were taken in the sub plot and IBA concentrations were in sub-sub plot. The average results of two years showed that planting dates, cutting types and IBA treatments and their interactions significantly affected all the growing and rooting parameters. Cuttings planted on July 16 revealed minimum days to sprouting (12.52), more number of sprouts (5.79), high sprout length (44.80 cm), more number of leaves (123), more number of roots (4.73), and plant survival percentage (61.78 %) as compared to the cuttings planted on August, 30 in both the years. Regarding cutting types, semi hard wood cuttings took minimum days to sprouting (16.91), more number of sprouts (4.30), sprout length (32.74 cm) number of leaves (113), number of roots (3.66) and higher plant survival percentage (40.81 %) in comparison to hard wood cuttings. Among various concentrations of IBA, application of IBA at the rate of 8000 ppm resulted in maximum number of sprouts (4.36), number of leaves (113), number of roots (3.61) and plant survival percentage (40.37 %) while minimum days to sprouting (17.07) were noticed at this treatment as compared to the control treatment. Years also exhibited significant impact for all the parameters. The interactive effect of planting dates, cutting types and various IBA concentrations had significant effect on all the parameters. Planting dates and cutting types interaction (PD × CT) significantly affected all the parameters except plant survival percentage. Therefore, it can be determined from the results that, in monsoon season planting of the semi-hard wood cuttings on July, 16 treated with 8000 ppm IBA concentration initiated better vegetative and root growth of Silvery under the climatic conditions of Peshawar, Khyber Pakhtunkhwa, Pakistan.
Received | March xx, 2021; Accepted |April 11, 2022; Published | September 15, 2022
*Correspondence | Nagina Zeb, Department of Horticulture, Faculty of Crop Production Sciences, The University of Agriculture, Khyber Pakhtunkhwa, Peshawar, Pakistan; Email: horti_nz@yahoo.com
Citation | Zeb, Z. and N.U. Amin. 2022. Influence of Planting dates, Cutting types and IBA Concentrations on Rooting of Silvery (Leucophyllum frutescens). Sarhad Journal of Agriculture, 38(4): 1160-1171.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.4.1160.1171
Keywords | Cutting types, IBA, Planting dates, Silvery (Leucophyllum frutescens)
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Silvery (Leucophyllum frutescens), an ornamental shrub and landscape plant belongs to Family Scrophulariaceae. It is called Silvery in USA. Other names given to Silvery include Purple Sage, White Sage, Silver Leaf and Texas Sage. It is an evergreen grayish color, foliaged shrub and grows up to 0.9-2.4 m long with a canopy of 0.9-1.8 m. It bears wavy look with 1.3-2.5 cm ovalish alternate leaves with silver pubescence. The leaves are soft and velvety with smooth margins. Flowers are five lobed tubular pink and rose-purple color with (1.3-2.5 cm) dimension. They appear singly in the leaf axil with spotted throat. Blooming normally occurs after summer rains and can cover the whole plant. Silvery bears small two valved capsules filled fruit at the base of flowers with tiny wrinkled seeds. Generally, silvery are planted in group which makes a good screen or hedge (Duever, 2000).
It’s perfect for large containers and as a shrub and perennial borders, grouping of several individuals make a hedge shape or pretty screen. Silvery prefers a well-drained potting mix. Usually, the bottom portion of cuttings is dipped in a rooting hormone to initiate and speed up the rooting phenomenon. Overwatering should be avoided because Silvery rots easily. Usually, it doesn’t need any fertilizer. If it’s necessary, a light application of general purpose fertilizer must be applied but not more than twice a year (Duever, 2000). It is so easy to propagate from cuttings that a new plant can be started nearly at any time of the year. Suggestions are usually in the favor of taking 4-inch (10 cm.) softwood cutting after the bloom comes to an end in summer, but hardwood cuttings can also be used when the plant is inactive in late fall or winter season.
The triumph of vegetative propagation by using cuttings basically depends on numerous factors, like genetic potential of root ability, physiological status of parent plant, hormonal balance season of the year, humidity, light and temperature (Soundy et al., 2008). Total carbohydrates and levels of nitrogen and C/N ratio has been stated to sway some species adventitious rooting (Rapaka et al., 2005). Cuttings having higher carbohydrates usually show better percentage of rooting (Hartmann et al., 2002b; Fachinello et al., 2005), but this can change considerably, depending on season of the year. Seasonal deviations are very common in rooting efficiency of woody plants, and the prime rooting season must be recognized for each species individually (Hussain et al., 2012a, 2014; Yamamoto et al., 2013). The plant growth regulators have significant role in plant growth and development. They play crucial role in cell division and cell expansion (Frick and Strader, 2018). Among these, IBA is the most active ingredient in plant propagation media, such as Root zone, to persuade adventitious roots in stem cuttings. Considering the inconsistency, rooting ability is IBA sensitive, depending on the species (Daskalakis et al., 2018). Keeping in mind the better growth and rooting capability of Silvery the current experiment has been designed to be performed in monsoon season using two types of cuttings being dipped in IBA concentrations and planted on different dates in monsoon season.
The major objectives of the experiment are to investigate various planting dates for better growth and success of Silvery and to find the best cutting type among the various under test cutting types for good root initiation. In addition to examine the optimum concentration of IBA for better rooting of the plant.
Materials and Methods
Experiment entitled “Influence of planting dates, cutting types and IBA concentrations on Silvery” was performed at ornamental nursery, Horticulture Department, The University of Agriculture, Peshawar in monsoon 2016 and repeated during monsoon 2017. During the experiment semi-hardwood and hardwood cuttings of Silvery were planted in Randomized Complete Block Design (RCBD) with split split plot arrangement. The whole trial was comprised of three factors and three replications. Ten cuttings per treatment were planted in polythene bags (9″x6″ in size). Five different concentrations of IBA i.e. 0 (control), 2000, 4000, 6000 and 8000 ppm were used for treating the cuttings before plantation. The total number of cuttings was 1500 for five planting dates with fifteen days interval.
Experimental factors
Factor A: Main plots
(Planting dates)
D1= July, 01
D2= July, 16
D3= July, 31
D4= August, 15
D5= August, 30
Factor B: Sub plots (Cutting types)
C1= Hardwood cutting
C2= Semi-hard wood cutting
Factor C: Sub-sub plot (IBA treatments)
A1= 0 ppm
A2= 2000 ppm
A3= 4000 ppm
A4= 6000 ppm
A5= 8000 ppm
The media was prepared by adding leaf mold, silt and garden soil with the ratio of 2:1:1 correspondingly. Perforated black polythene bags of 9x6″ were filled with the above-mentioned media mixture. Then about 10-15 cm long semi-hardwood and hardwood cuttings were taken from healthy and vigorous Silvery plants. Slanting cuts were given to the cuttings in order to ensure easy insertion, exposure of more cambium area and minimum disease attack. Prior to insertion in media these cuttings were dipped in various concentrations of IBA. Each cutting was left with 4 buds and 8 leaves and planted in the bags, while the rest was removed. Irrigation and other cultural practices were constant throughout the experiment.
Weather data of Peshawar (Experimental site)
The Figure 1 is showing maximum and minimum temperature and total rainfall of investigational site during the time period of the whole experiment.
Physico-chemical analysis of soil
Prior to the plantation of cuttings of Silvery in each year, 5 different soil samples were collected from the soil used for experiment. The samples were assorted together to make a compound sample. The compound sample was then dried in air and sieved to remove plant deposits, stones, pebbles or other unessential materials with a mesh of 2mm. various physico-chemical characteristics of the soil were evaluated and are shown in Table 1, which shows that the soil texture is silty loam, alkaline in reaction, containing organic matter content (1.0 %) with a total N content of 0.05 dSm-1 and has a mean soil pH of 8.3.
Parameters studied
The following data on different growth parameters regarding Silvery plant propagated through cuttings were recorded. The data was collected on five randomly selected plants.
Days to sprouting: Total number of days to sprouting cutting-1 was totaled for each treatment in all replications and average was driven out.
Number of sprouts: The sprout number of all survived plants was added up for every treatment and then the mean was calculated.
Table 1: Physico-chemical characteristics and analysis of soil during 2016 and 2017.
|
Property |
Unit |
2016 |
2017 |
|
Clay |
% |
24.0 |
26.0 |
|
Silt |
% |
48.0 |
44.0 |
|
Sand |
% |
28.0 |
30.0 |
|
Textural class |
----- |
Silt loam |
Silt loam |
|
pH (1: 5) |
----- |
8.4 |
8.2 |
|
EC (1: 5) |
ds m-1 |
0.35 |
0.35 |
|
TSS |
% |
0.112 |
0.112 |
|
Organic matter |
% |
1.0 |
1.0 |
|
Lime (CaCO3) |
% |
4.5 |
5.0 |
|
Total nitrogen |
mg kg -1 |
0.050 |
0.051 |
|
Total phosphorous |
mg kg -1 |
19.7 |
25.1 |
Sprout length (cm): Length of the sprouts of all subsisted plants was measured with the help of measuring tape from the base to the tip of the lengthiest sprout for all the treatments in all replications and then the mean was calculated.
Number of leaves: The total number of leaves of all the successful grown plants was totaled for each of the treatment and then the mean was calculated.
Number of roots: All of the survived plants were excavated carefully; the roots were gently washed with tape water and then the initiated roots were counted.
Plant survival percentage (%): Percentage of survived cuttings was worked out by using following formula:
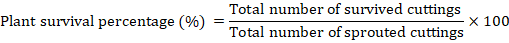
Experimental design and statistical analysis
The recorded data was arranged as Randomized Complete Block Design (RCBD) with split split plot arrangement and subjected to Analysis of Variance technique as specified by Steel and Torrie (1980) and then it was analyzed using statistical software Statistix 8.1 (Statistix-8 Analytical Software. 2003). For instance the data found significant, least significant difference (LSD) test was applied for mean judgments.
Table 2: Days to sprouting, number of sprouts and sprout length (cm) as affected by planting dates, cutting types and IBA concentrations in monsoon season.
|
Planting dates (PD) |
Days to sprouting |
Number of sprouts |
Sprout length (cm) |
|
July 01 |
14.93 e |
4.59 b |
39.04 b |
|
July 16 |
12.52 d |
5.79 a |
44.80 a |
|
July 31 |
17.20 c |
3.92 c |
31.57 c |
|
August 15 |
19.71 b |
3.31 d |
22.10 d |
|
August 30 |
23.72 a |
2.53 e |
18.47 e |
|
LSD (0.05) |
0.32 |
0.21 |
0.55 |
|
Cutting types (CT) |
|||
|
Hardwood cutting |
18.32 a |
3.75 b |
29.66 b |
|
Semi-hardwood cutting |
16.91 b |
4.30 a |
32.74 a |
|
LSD (0.05) |
0.18 |
0.09 |
0.32 |
|
IBA concentrations (ppm) |
|||
|
Control |
18.17 a |
3.71 d |
29.98 e |
|
2000 |
17.89 b |
3.93 c |
30.51 d |
|
4000 |
17.60 c |
3.95 c |
31.14 c |
|
6000 |
17.34 d |
4.18 b |
31.86 b |
|
8000 |
17.07 e |
4.36 a |
32.49 a |
|
LSD (0.05) |
0.22 |
0.17 |
0.51 |
|
Years (Y) |
|||
|
2016 |
17.37 b |
4.26 a |
32.10 a |
|
2017 |
17.85 a |
3.78 b |
30.29 b |
|
Interactions |
|||
|
Y × PD |
NS |
* |
* |
|
Y × CT |
NS |
NS |
** |
|
Y × IBA |
NS |
NS |
NS |
|
PD × CT |
** |
** |
* |
|
PD × IBA |
NS |
NS |
NS |
|
CT × IBA |
NS |
NS |
NS |
|
Y × PD × CT |
NS |
NS |
** |
|
Y × PD × IBA |
NS |
NS |
NS |
|
Y × CT × IBA |
NS |
NS |
NS |
|
PD × CT × IBA |
NS |
NS |
NS |
|
Y × PD × CT × IBA |
NS |
NS |
NS |
Means followed by the different letters are significantly different. *, ** show probability level of 5 % and 1 %, respectively. NS means non-significant.
Results and Discussion
Days to sprouting
Data about days to sprouting of silvery cuttings in retort to different planting dates (PD), cutting types (CT) and IBA concentrations is shown in Table 2. Days to sprouting were significantly affected by planting dates, cutting types, IBA concentrations and year as source of variation. The interaction was not found significant except PD × CT (Figure 2).
The days to sprouting (12.52) were less; when the cuttings were planted on July 16, while highest days (23.72) were recorded in the plants planted on August 30. Semi hard wood cutting significantly took less days to sprouting (16.91) as compared to (18.32) in hard wood cuttings. Among various concentrations of IBA, the highest concentration i.e. 8000 ppm decreased the days to sprouting of the cuttings (17.07) in comparison to the absence of IBA (0 ppm) (18.17). The mean data displayed that days to sprouting (17.37) in 2016 were less as compared to 2017, which was recorded as 17.85. The only significant interaction between PD × CT showed that both the hardwood and semi-hardwood cuttings showed declined trend noted on July, 16 plantation and increasing trend afterward, the maximum days to sprouting were recorded on August, 30. Also the semi-hardwood cutting exhibited less variability to days to sprouting as compared to hardwood cutting (Figure 2).
The results of current experiment are like this might be due to exogenous auxin application to the cuttings, which might have brought early breakage of bud dormancy and resulted in early bud sprouting (Padekar et al., 2018). The earliest sprouting on semi hardwood cuttings might possibly be due to more absorption of nutrients and uptake of water from roots towards shoots (Mehta et al., 2016). The results of present investigations are accordance with the finding of Mishra and Sahu (1983) and Taslim et al. (1992) in Kartoli. Hartmann et al. (1990b) also reported that, in many vegetatively propagated species, mature, lignified wood cuttings are more difficult to form roots than newly formed stem system. Chandramouli (2001) also suggested that raise in the concentration of IBA significantly decreased the number of days to first sprouting of cuttings and earliness in sprouting might be due to well consumption of nitrogen, stored carbohydrates and other aspects with assistance of growth regulators.
Number of sprouts
The results presented in mean Table 2 revealed that different planting dates, cutting types and various IBA concentrations had significant variations. The interactions are shown in Figure 3a and 3b. The interaction between Y × PD and PD × CT also showed significant effect on number of sprouts.
Among planting dates, maximum number of sprouts (5.79) was noticed on July, 16 planting, while minimum sprouts (2.53) were counted on August, 30 planting date’s plants. Concerning cutting types, maximum number of sprouts (4.30) were counted in the semi hard wood cuttings, while (3.75) number of sprouts was observed in hard wood cuttings. In case of various IBA concentrations, maximum number of sprouts (4.36) was counted in the cuttings dipped in 8000 ppm concentrated IBA solution, while with no IBA treatment, the cuttings produced less (3.71) number of sprouts. Year was also found significant. Number of sprouts cutting-1 was maximum (4.26) during 2016, as compared to 2017 (3.78). The Y × PD interaction revealed that, an increasing trend of number of sprouts was observed on July, 16 and after that, a decline was observed and minimum number of sprouts were counted on August, 30 in both the years 2016 and 2017 (Figure 3a). The interaction between PD × CT presented in the Figure 3b showed that the standard error of both of the cutting types at different planting dates showed increasing trend of number of sprouts on July, 16 and decreased afterwards, recorded minimum number of sprouts on August, 30. Also in the semi-hardwood cutting type, the number of sprouts is less variable as compared to hardwood cutting type.
The maximum number of shoots in IBA treated cuttings might be due to its effect on cell wall plasticity, which accelerates cell division stimulates callus development and growth. By using growth regulators, the increase in number of shoot formation, might be because of the vigorous root system which made uptake of the nutrients easy due to power of IBA application which influenced the cell division in vascular cambium, cell enlargement and control of differentiation into diverse types of cambium occasioning in raise of number of shoots (Devi et al., 2016). Results of present findings are in agreement with findings reported in pomegranate by Dhillon and Sharma (1992) and in sweet lime by Kumar et al. (2004). Singh and Chouhan (2016) also investigated maximum number of sprouted cuttings (6.62) under mid-august planting time and IBA concentration significantly increased the average maximum number of sprouted cuttings (4.47) at the rate of 2000 ppm in Phalsa cuttings. Padekar et al. (2018) indicated that, IBA at 750 ppm recorded significantly maximum number of shoots per cutting i.e. (2.32) and regarding the influence of types of cutting on number of shoots per cutting, the semi hard wood cutting recorded significantly maximum number of shoots per cutting (3.11).
Sprout length (cm)
Mean values of the data elaborated in Table 2 revealed that planting dates, cutting types and IBA concentrations had significantly influenced sprout length of the cutting. Year as a source of variation also showed significant variation. The interactions between Y × PD, Y × CT and PD × CT were also found significant and were presented in Figure 4a, 4b and 4c. Among planting dates, the lengthy sprouts (44.80 cm) were measured in the cuttings planted on July 16, while shortest sprout length (22.10 cm) and (18.47 cm) was measured in cuttings planted on August, 15 and August, 30, respectively. Regarding cutting types, maximum sprout length (32.74 cm) was measured in semi hard wood cuttings while minimum sprout length (29.66 cm) was testified in hard wood cuttings. IBA concentrations also significantly increased the sprout length to (32.49 cm) at 8000 ppm IBA solution from (29.98 cm) in control treatment (0 ppm). In case of year, a significant variation was observed in 2016 and 2017 regarding sprout length. Lengthy sprouts (32.10 cm) were observed in 2016 as compared to (30.29 cm) in 2017. The interaction between Y × PD showed an increasing trend for sprout length in both the years 2016-17 on planting date of July 16, and then a downward drift was observed on August, 30 among other planting dates. (Figure 4a). Y × CT illustrated in Figure 4b, indicated that in both the years, different cutting types showed lower trend of sprout length in hardwood cutting type and then increased, recorded maximum sprout length in semi-hardwood cutting type. PD × CT described that an increasing trend of sprout length on July, 16 for both the hardwood and semi-hardwood cutting types. After maximum length, a decline was observed and minimum sprout length was measured on August, 30, in case of both the cutting types (Figure 4c).
Auxins activated shoot growth which might have triggered hydrolysis and translocation of carbohydrates, accumulation of nitrogenous elements at the base of the cuttings and caused cell acceleration, elongation and division (Singh et al., 2003). Padekar et al. (2018) also reported that, semi hardwood cuttings treated with IBA and planted in Soil + Sand + FYM (1:1:1) showed maximum length of main shoot. Singh and Chouhan (2016) observed longest sprout (5.78 cm) in phalsa by planting the cuttings in mid-August planting time and with IBA at the rate of 2000 ppm IBA. Padekar et al. (2018) also reported in their experiment that among the effect of IBA concentrations on length of main shoot, the IBA at 750 ppm recorded maximum length of main shoot.
Number of leaves
Data regarding number of leaves is presented in Table 3. Planting dates, cutting types, various IBA concentrations and year as a source of variation, showed significant effect on number of leaves. The interactions between Y × PD and PD × CT also showed significant effect and are presented in Figure 5a and 5b.
Mean values of the data revealed that maximum number of leaves (123) was counted in the cuttings planted on July 16, while minimum leaves (101) were counted in the cuttings planted on August 30. Regarding cutting types, it was perceived that semi hard wood cutting showed maximum number of leaves (113) as compared to hard wood cuttings (110). It was observed that among various IBA solutions, 8000 ppm showed maximum number of leaves (113) in relation to control (110). Regarding years, more number of leaves (113) was counted during 2016 in comparison to (110.07) in 2017. Interaction between Y × PD is given in (Figure- 5 (a), which indicated that in both the years i.e. 2016 and 2017, an increasing trend with regard to number of leaves was observed on July, 16 and then a decreased line was observed towards August 30, recorded the lowest number of leaves. It is obvious from the interaction of PD × CT that the standard error of both cutting types at different planting dates exposed upper trend of number of leaves on July, 16 and decreased afterwards, and the lower number of leaves was recorded on August, 30. Moreover, the semi-hardwood cutting had more uniformity as compared to hardwood cutting (Figure 5b).
The current experiment was performed during the monsoon season which supports high humidity and therefore reducing the heat capacity on cuttings hence allowing the utilization of supreme light conditions to rise photosynthesis (Hartmann et al. 1990a; Acquaah, 2005). Leaves are the primary photosynthetic organ which captures sunlight for the process of photosynthesis towards the growth and subsequent development of the plant (Stancato et al., 2003). It is vital to maintain high humidity levels while rooting vegetative stem cuttings in order to reduce water loss due to transpiration (Stancato et al., 2003). IBA also contributed towards more leaves on a plant. The reason behind this could be that the application of IBA leads to the formation of root initials and thus root formation and finally into absorbance of more amount of nutrient from soil that led to higher leaf number (Mehta et al., 2016). Similar results like the current research were also reported by Singh and Chouhan in 2016 that maximum number of leaves (14.57) were counted in mid-August and with IBA application of 8000 ppm, maximum number of leaves (12.60) was reported in the cuttings of Phalsa.
Table 3: Number of leaves, number of roots and plant survival percentage (%) as affected by planting dates, cutting types and IBA concentrations in monsoon season.
|
Planting dates (PD) |
Number of leaves |
Number of roots |
Plant survival percentage (%) |
|
July 01 |
115 b |
3.92 b |
47.67 b |
|
July 16 |
123 a |
4.73 a |
61.78 a |
|
July 31 |
111 c |
3.41 c |
36.08 c |
|
August 15 |
107 d |
2.95 d |
26.27 d |
|
August 30 |
101 e |
2.27 e |
19.43 e |
|
LSD (0.05) |
0.75 |
0.14 |
2.02 |
|
Cutting types (CT) |
|||
|
Hardwood cutting |
110 b |
3.25 b |
35.68 b |
|
Semi-hardwood cutting |
113 a |
3.66 a |
40.81 a |
|
LSD (0.05) |
0.25 |
0.08 |
1.44 |
|
IBA concentrations (ppm) |
|||
|
Control |
110.25 e |
3.26 d |
36.12 d |
|
2000 |
110.82 d |
3.42 c |
36.85 cd |
|
4000 |
111.34 c |
3.47 bc |
38.25 bc |
|
6000 |
111.91 b |
3.52 ab |
39.65 ab |
|
8000 |
112.62 a |
3.61a |
40.37 a |
|
LSD (0.05) |
0.31 |
0.09 |
2.00 |
|
Years (Y) |
|||
|
2016 |
112.71 a |
3.68 a |
39.29 a |
|
2017 |
110.07 b |
3.23 b |
37.20 b |
|
Interactions |
|||
|
Y × PD |
** |
* |
NS |
|
Y × CT |
NS |
NS |
NS |
|
Y × IBA |
NS |
NS |
NS |
|
PD × CT |
** |
** |
NS |
|
PD × IBA |
NS |
NS |
NS |
|
CT × IBA |
NS |
* |
NS |
|
Y × PD × CT |
** |
* |
NS |
|
Y × PD × IBA |
NS |
NS |
NS |
|
Y × CT × IBA |
NS |
NS |
NS |
|
PD × CT × IBA |
NS |
NS |
NS |
|
Y × PD × CT × IBA |
NS |
NS |
NS |
Means followed by the same letters are not significantly different. *, ** show probability level of 5 % and 1 %, correspondingly. NS stand for non-significant.
Number of roots
Mean data set in Table 3 indicated that there is found significant difference between planting dates, cutting types, IBA concentrations and year of planting on number of roots. The interactions among Y × PD, PD × CT and CT × IBA were also found significant and presented in Figure 6a, 6b and 6c.
Maximum number of roots (4.73) was counted in the plants of July, 16, while less number of roots (2.27) was witnessed in cuttings of August, 30. Regarding cutting types, more number of roots (3.66) was formed by semi hardwood cuttings, as compared to hardwood cuttings (3.25). As for as various concentrations of IBA is concerned, it was apparent from the results that IBA at the rate of 8000 ppm showed highest number of roots (3.61), followed by 6000 ppm (3.52), when compared to control (0 ppm), exhibiting least number of roots (3.26). Year as a source of variation was also found significant as far as number of roots is concerned. More number of roots (3.68) was observed in 2016 as compared to (3.23) in the year 2017 where number of roots was decreased. Figure 6a, showing significance of the interaction between Y × PD, that in both the years 2016 and 2017, an increasing trend of number of roots was recorded on July, 16 among different planting dates and then a decreased impression of number of roots was seen on August, 30 as compared to other planting dates. Interaction between PD × CT as displayed in Figure 6b revealed that the standard error of both cutting types at different planting dates showed high trend of number of roots on July, 16 and decreased afterwards, recorded less roots on August, 30. Moreover, the semi-hardwood cutting had low variability as compared to hardwood cutting type. It is also evident from the interactive effect of CT × IBA that a rising trend regarding number of roots was observed with 8000 ppm IBA concentration, used for both hardwood and semi-hardwood cutting types and a declined trend was seen on control treatment of IBA. Moreover, semi-hardwood cuttings showed more reliability as compared to hardwood cutting Figure 6c.
During the propagation process, cuttings require water to prevent withering (death) and for the processes like photosynthesis, which influence root growth and development (Owen, 2018). Auxin standardizes sundry physiological processes that include apical dominance, cell division and root initiation. Auxin induces adventitious root development in cuttings that are used to propagate the plants. Stem cuttings of many plants, when are dipped or coated with small amounts of auxin, develop roots in greater numbers and very quickly (Susaj et al., 2012). After cell absorption, NAA and IAA may cause a fairly swift increase in cell wall extensibility in fresh stems (Vanzile, 2011).
The current experiment’s results support the findings of Khan et al. (2006) who mentioned that less mature a plant, normally the easier it is to root a cutting, also the less mature the growth stage of a plant i.e. softwood, the more effortlessly it can lose water, wilt and die. Summing up, (Day and Loveys 1998; Dole and Wilkins, 2005) also stated that rooting success in the majority of ornamental plants is dependent on physiological phase of parent plant. Rooting also diverges with cutting type, the rooted species and environmental circumstances (Ibironke, 2013). Variations in environmental conditions of mother plants can also affect the ability to root, age of the mother plant also plays a vital part in root ability of the cutting (Low and Hackett, 1981). Our findings are in accordance with Ibironke (2013). Nutritional status of a plant is a significant element in successful rooting of a cutting (Janick, 1986). The vigorous rooting of semi hardwood cuttings facilitated the cuttings to engross supplementary nutrients and produce more sprouts and leaves and improves the plant vigor. It was also seen through other investigations on Phalsa cutting that maximum rooting percentage was observed in the period of mid-August with the 5 second dipping of the cuttings in 2000 ppm IBA (Singh and Chouhan, 2016). However, according to Low and Hackett, 1981 cuttings of jojoba plant rooted 100 percent in both July and August with 4000 ppm IBA treatment, but the features of root system formed in August was significantly lower than in July; Evans and Blazish (1999) perceived that possibly the best time to take cuttings is the beginning of the rainy season.
Plant survival percentage (%)
Table 3 showed the mean data of plant survival percentage displaying significant difference among various planting dates, cutting types, IBA concentrations and year. None of the interaction was found significant. The maximum plant survival (61.78 %) was observed in the cuttings planted on July, 16 i.e. monsoon season, whereas (19.43 %) plant survival was witnessed in the cuttings planted on August, 30. Cutting types also had significant response on plant survival percentage. Semi hard wood cuttings exhibited more survival percentage (40.81 %) as compared to the hard wood cuttings (35.68 %). Significance of IBA treatments indicated that, application of IBA at the rate of 8000 ppm resulted in maximum survival (40.37 %), followed by (39.65 %) with the application of 6000 ppm IBA, while minimum survival (36.12 %) was recorded at control (0 ppm concentration). Regarding year, supreme survival (39.29 %) was observed during 2016 in all the planting dates as compared to (37.20 %) in 2017.
The better percentage of cutting’s survival might be ascribed with optimum time and IBA treatments aid to better root growth which amplified the absorption and transportation of nutrients from soil resultantly boost up plant metabolic processes (Singh, 2001). The results of current experiment are also in line with the data of the experiment of Padekar et al. (2018) who reveals that, the semi hardwood cutting recorded significantly maximum survival percentage of 61.67 % as compared to the basal portion of the plant of momordica. Among the effect of IBA concentrations on survival percentage of momordica, the IBA at highest level of 10,000 ppm recorded significantly maximum survival percentage i.e. 70.56 %.
Application of auxin is hereby found to augment the histological features i.e. callus and tissue formation and differentiation of vascular tissues (Singh and Chouhan, 2016). Majeed et al. (2009) observed highest rooting rate i.e. 50% in the cuttings of Aesculus indica, treated with 2000 ppm IBA concentration. It is due to auxin mode of action which may have caused hydrolysis and translocation of carbohydrates and nitrogenous substances at the lower ends of cuttings and caused augmented cell elongation and division in appropriate environmental conditions (Hartmann et al., 2007).
Conclusions and Recommendations
From the current experiment and analyzed data it was concluded that, planting the Semi hard wood cuttings of Silvery in mid monsoon season and dipped in 8000 ppm IBA solution produced better growing and rooting response. It is recommended that plantation of Silvery cutting should be carried out during monsoon (July, 16) for maximum sprouting, better rooting and high survival percentage under the agro-climatic conditions of Peshawar. Semi hard wood cuttings of Silvery must be used to obtain optimum vegetative and rooting growth. Obtaining healthier and good roots of Silvery, IBA at the rate of 8000 ppm should be applied.
Novelty Statements
Silvery being an important landscape plant is a difficult plant to root. Current study played a helpful role in overcoming of this problem of the concerned plant by using the current propagation technique.
Author’s Contribution
Nagina Zeb: Conducted the research, Analysis and composed this manuscript.
Noor ul Amin: Provided guidelines during the whole study and experiments.
Conflict of interest
The authors have declared no conflict of interest.
References
Acquaah, G. 2005. Horticulture: Principles and Practices, Third Edition, Upper Saddle River, Pearson Education Incorporated Hall New Jersey. pp: 367.
Chandramouli, H. 2001. Influence of growth regulators on the rooting of different types of cuttings in Bursera penicilliata (DC) Engl. A M.Sc. (Agri.) thesis, University of Agricultural Sciences, Bangalore.
Daskalakis I, K. Biniari, D. Bouza and M. Stavrakaki. 2018. The effect that Indolebutyric acid (IBA) and position of cane segment have on the rooting of cuttings from grapevine rootstocks and from Cabernet franc (Vitis vinifera L.) under conditions of a hydroponic culture system. Sci. Horti., 227:79-84. https://doi.org/10.1016/j.scienta.2017.09.024
Day, J. S. and B. R. Loveys. 1998. Propagation from cuttings of two woody ornamental Australian shrubs, Boronia megastigma Nees. (Brown boronia) and Hypocalymma angustifolium Endl. (White myrtle). Aust. J. Exp. Agric., 38: 201-206. https://doi.org/10.1071/EA97075
Devi, J., P. Bakshi, V.K. Wali, K. Kour and N. Sharma. 2016. Role of auxin and dates of planting on growth of cuttings raised plantlets of phalsa (Grewia asiatica L.). The Bioscan., 11: 535-537.
Dhillon, W.S. and Sharma K. K. 1992. Effect of IBA on rooting of cuttings in pomegranate (Punica granatum L.). J. Res. Punjab. Agric. Uni., 29: 350-353.
Dole, J.M. and H.F. Wilkins. 2005. Floriculture: principles and species. 2nd Edi. Prentice Hall, Upper Saddle River.
Duever, L.C. 2000. Leucophyllum frutescens. http://dx.doi.org/floridata.com.
Evans. E. and F. Blazich. 1999. Plant Propagation by Stem Cuttings. NC State Extension Publications.
Fachinello, J.C., A. Hoffman and J.C. Nachtigal. 2005. Propagation of frutiferas brasilia, DF: Embrapa Informacao Techno. pp. 221-224.
Frick, E.M. and L.C. StraderRoles for IBA derived auxin in plant development. J. Exp. Bot., 69(2): 169-177. https://doi.org/10.1093/jxb/erx298
Hartmann, H.T., Kester, D.E. and Davies, F.T. and J.R. 1990a. Plant Propagation: Principles and Practices, 5th Edition, Prentice Hall International Editions. Englewood Cliffs, New Jersey, USA.
Hartmann, H.P., D.E. Kester and J.T. Davies, 1990b. Plant Propagation: Principle and Practices. Prentice-Hall, New Jersey. Pp: 632-649.
Hartmann, H.T., D.E. Kester, F.T. Davies and R.L. Geneve. 2002b. Plant Propagation Principles and Practices, Seventh Edition, Prentice Hall, New Jersey, pp. 367-374.
Hartmann, H.T., D.E. Kester, F.T. Devies and R.L. Geneve. 2007. Plant Propagation Principles and Practices. Seventh Edition, Prentice Hall of India Pvt. Ltd., New Delhi.
Hussain, S., S. Fareed, S. Ansari, M.A. Rahman, I.Z. Ahmad and M. Saeed. 2012a. Current approaches toward production of secondary plant metabolites. J. Pharm. Bio Allied Sci., 4(1): 10-20. https://doi.org/10.4103/0975-7406.92725
Hussain, I.A.M. Assis, L.Y. Yamamoto, R. Koyama and S.R. Roberto. 2014. Indole butyric acid and substrates influence on multiplication of blackberry Xavante Ciencia Rural, Santa Maria, 44(10):1761-1765. https://doi.org/10.1590/0103-8478cr20131204
Ibironke, O.A. 2013. The Effects of Cutting Types and Length on Rooting of Duranta repens in the Nursery. Glob. J. Human Soc. Sci. Geograph., Geo-Sci., Environ. Disast. Manage., 13(3): 1-4.
Janick, J. 1986. Horticultural Science. 4th edn. W.H. Freeman, New York. https://doi.org/10.1002/9781118060810
Khan, M.S, R.U. Khan and K. Waseem. 2006. Effect of some auxins on growth of Damask Rose Cutting in different growing media. J. Agric. Soc. Sci., 2(1): 13-16.
Kumar, S., H.S. Shukla and S. Kumar. 2004. Effect of IBA (Indole butyric Acid) and PHB (p-hydroxy benzoic acid) on the regeneration of sweet lime (Citrus limettioides Tanaka) through stem cuttings. Progressive Agric., 4: 54-56.
Low, C.B. and W.P. Hackett. 1981. Vegetative propagation of Jojoba. California Agric., 35: 12-13.
Majeed, M., M.A. Khan and A.H. Mughal. 2009. Vegetative propagation of Aesulus indica through stem cuttings treated with plant growth regulators. J. For. Res., 20(2): 171-173. https://doi.org/10.1007/s11676-009-0031-1
Mehta, N.S., S.S. Bhatt, J. Kumar, A. Kotiyal, D.C.D. Mehta, N.S., S.S. Bhatt, J. Kumar, A. Kotiyal and D.C. Dimri. 2016. Effect of IBA on vegetative growth and multiplication rate in stem cuttings of pear rootstocks. Horti. Flora. Res. Spectrum, 5(3): 242-245.
Maity, S.C. and Mitra, S.K. 1990. Litchi. In: Fruits: Tropical and Subtropical. Eds. Bose and Mitra, Naya Prokash, Calcutta. pp. 428.
Mishra, K.C. and R.P. Sahu. 1983. Large scale cultivation of small bitter gourd: Problems and possibilities. Indian Horti., 28(3): 5-8.
Owen, W.G. 2018. Moisture management during vegetative cutting propagation. Michigan State University Extension.
Padekar, V.J., V.K. Garande, S.S. Dodake, S.V. Sawant, U.S. Shinde, P.N. and Sonawane, R.D. 2018. Effect of IBA, types of cutting and rooting media on sprouting, survival percentage and growth of cuttings of Kartoli (Momordica dioica). Int. J. Curr. Microbiol. App. Sci., 7(10): 1246-1260. https://doi.org/10.20546/ijcmas.2018.710.140
Rapaka, V.K., B. Bessler, M. Schreiner and U. druege. 2005. Interplay between initial carbohydrate availability, current photosynthesis, and adventitious root formation in Pelargonium cuttings. Plant Sci. Ireland, 168: 1547-1560. https://doi.org/10.1016/j.plantsci.2005.02.006
Steel, R.G.D. and J.H. Torrie. 1980. Principals and procedures of statistics. 2nd ed. McGraw Hill, New York.
Singh, A.K. 2001. Effect of wood type and root promoting chemical on rooting of Bougainvillea peruviana L. Adv. Horti. For., 8: 179-184.
Singh, K.K and J.S. Chouhan. 2016. The effect of different times collecting cutting, growing conditions and Auxin treatments of the rooting in Phalsa (Grewia asiatica L.) stem cutting under valley condition of Garahwl. Plant Arch., 16 (2):781-788.
Stancato, G.C, F.F.A. Aguiar, S. Kanashiro and A.R. Tavares. 2003. Rhipsalis grandiflora haw propagation by stem cuttings. Sci. Agric., 56:185-190.
Susaj, E., L. Susaj and I. Kallco. 2012. Effect of different NAA and IBA concentrations on rooting of vegetative cuttings of two rose cultivars. Res. J. Agric Sci., 44(3):121-127.
Soundy, P., K.W. Mpati, E.S. Du-Toit, F.N. Mudau and H.T. Araya. 2008. Influence of cutting position, medium, hormone and season on rooting of fever tea (Lippia javanica L.) stem cuttings. Med. Aromatic Plant Sci. Biotechnol. Isleworth, 2(2):114-116.
Taslim, A., P.T. Shukla, U.P. Chovatia and J.A. Makati. 1992. Propagation of kartoli through cuttings by use of IBA. Gujrat agri. Univ. Res. J., 17 (2): 99-101.
Vanzile, J. 2011. Using rooting hormone to propagate plants. http://houseplants.about.com/od/propagatingyourplants/RootHormone.htm
Yamamoto, L.Y., R. Koyama, W.F.S. Borges, L.E.C. Antunes, A.M. Assis and S.R. Roberto. 2013. Substrates on rooting of blackberry Xavante herbaceous cuttings. Ciencia Rural, Santa Maria, 43(1):15-20. https://doi.org/10.1590/S0103-84782012005000135
To share on other social networks, click on any share button. What are these?







