In vivo Anti Inflammation Studies of Novel 1, 2, 5 Oxadiazole Sulfonamide Hybrids
In vivo Anti Inflammation Studies of Novel 1, 2, 5 Oxadiazole Sulfonamide Hybrids
Hafiz Adnan Ahmad1,2*, Muhammad Aslam2, Salman Gul2, Tariq Mehmood2 and Munawar Ali Munawar2
1Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, Department of Chemistry and Materials Science, Northwest University, Xi’an 710069, People’s Republic of China
2Institute of Chemistry, University of the Punjab, Lahore 54590, Pakistan
ABSTRACT
In present study we designed and synthesized a novel series of 1,2,5-oxadiazole-sulfonamide hybrids (4a-4j) in search of more potent anti-inflammatory agents. Title compounds were synthesized by chlorosulfonation of 3,4-diphenyl-1,2,5-oxadiazole (3) followed by condensation with amines and all derivatives were obtained in moderate to good yield. All synthesized hybrids were characterized with spectroscopic techniques and further evaluated for anti-inflammatory potential. The synthesized hybrids were screened in vivo by employing carrageenan-induced paw edema method at 10 mg/kg dose. Among all derivatives, two compounds 4g and 4b displayed better anti-inflammation potential than standard drugs celecoxib and indomethacin. While the anti-inflammation profile of compounds 4h, 4e and 4f during in vivo screening was also comparable to celecoxib and indomethacin. The structure-activity relationship (SAR) was also discussed with the reference of substituent nature. Present results showed that newly synthesized hybrids have significant anti-inflammatory potential and might be played an important role to the development of more potent anti-inflammation agents in future.
Article Information
Revised 30 July 2020
Accepted 12 January 2021
Available online 24 June 2021
(early access)
Published 26 February 2022
Authors’ Contribution
MAM designed and supervised the research. HAA synthesized and purified the compounds. HAA and SG performed spectral studies. MA and TM did biological studies.
Key words
Anti-inflammatory agent, Sulfonamide, NSAIDs, Oxadiazole
DOI: https://dx.doi.org/10.17582/journal.pjz/20200601040658
* Corresponding author: adnan.ahmad.pu@hotmail.com
0030-9923/2022/0003-1355 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
INTRODUCTION
Inflammation is a common phenomenon in the humans as well as animals and affected the life of millions of people every year. It is a complex process and produced because of defensive immune system response towards the injury of cellar or molecular component of host due to the attack of body enemy like bacteria, viruses etc. or burning (Medzhitov, 2008). In this way immune system removed harmful stimuli and maintained tissue homeostasis. Mild and acute inflammation vanished shortly and not dangerous for body. However longer duration of chonic inflammation not beneficial for the body and can cause various disease such as cancer, cardiovascular disease, osteoarthitis and neurodegenerative disorder (Grivennikov et al., 2010; Medzhitov, 2010; Nasef et al., 2017). Different kind of therapeutic agents are available to (employed) to control inflammatory disorder and pain like corticosteroids, selective cyclooxygenase-2 (COX-2) inhibitor, glucocoriticoids, immune suppressive agents and non-steroidal anti-inflammatory drugs (NSAIDs) (Caro et al., 2017; Hodge and Hodge, 2019).
Among all these NSAIDs are extensively studied and widely using in a daily life as a primary prefer drugs for the treatment of different kind of inflammation and pain (Praveen and Knaus, 2008; Sehajpal et al., 2018). Furthermore, literature survey revealed that NSAIDs are also helpful in the treatment of cancer. NSAIDs prevent the biosynthesis of pro-inflammatory prostaglandins (PGs) though the inhibition of cyclooxygenase (COX) that are rate-limiting enzymes in this process and exist in thee isoforms COX-1, COX-2, COX-3 (Ricciotti and FitzGerald, 2011; Dhingra et al., 2017).
However, the majority of available NSAIDs in market have their own potential side effects like bleeding, cardiovascular toxicity, decrease bone healing, gastrointestinal ulceration and hepatic toxicity due to non-selective inhibition of COX enzymes (Ricciotti and FitzGerald, 2011; Scheiman, 2016; Fanelli et al., 2017; Marzano et al., 2019). COX-1 is the constitutive isoform of COX enzyme and known as housekeeping enzyme, while newly discovered COX-3 is a splice variant of COX-1 and not take part inflammation process like COX-1. In fact, its COX-2 who is responsible for inflammation due to the catalysis of PGs synthesis. Therefore, the inhibition of selective COX-2 enzyme is important and significantly decrease the side effects of NSAIDs (Fabiola et al., 2001; Papanagnou et al., 2015).
The extensive research in this area during last three decades leads to the development of large number of COX-2 selective inhibitor. In general terms this class of compounds known as coxibs and most of them have five membered heterocyclic cores along with two aryl substitutions at adjacent position of the central core. Among all coxibs most famous coxibs also have sulfonamide functionality on one aryl group at pera position. Celecoxibs and rofecoxib belongs to the first generation of this class and launched in market in 1999 as selective COX-2 inhibitors after the approval of FDA (Chan et al., 1999; Silverstein et al., 2000). After that, several others coxibs were introduced for the treatment inflammation such as valdecoxib, parecoxib and etoricoxib (Riendeau et al., 2001; Padi et al., 2004). However, some coxibs latterly withdraw from market due to their adverse side effects like cardiovascular associated risk (Burnier, 2005; Praveen and Knaus, 2008; Thomas et al., 2017; Radi and Khan, 2019).
Therefore, the development of new and more effective active anti-inflammatory agent without or with insignificant side effects are still challenging for researchers. The scaffold of well-known coxibs are more suitable to design new drugs with the aim of better result and least side effects. Further in many structure activity relations studies it has been observed that the presence of sulfonamide functionality at one aryl group attributed the anti-inflammatory activity of the coxibs (Carullo et al., 2017). However, most of the known coxibs have primary sulfonamide functionality in their scaffold except parecoxib which is a secondary sulfonamide derivative of valdecoxib. Further, hardly in a very few reported studies the central corer of famous coxibs have been replaced with oxadiazole scaffold (Mange et al., 2007).
To the best of our knowledge, to date, no one has investigated the anti-inflammatory potential of oxadiazole analogue of valdecoxib by introducing secondary and tertiary sulfonamide functionality at meta position (Fig. 1). In continuation to our quest for the discovery of new and more potent drug candidates based on an important pharmacophore (Batool et al., 2018; Chaudhy et al., 2019; Fauzia et al., 2019) in this study, we designed and synthesized a novel series of 1, 2, 5-oxadiazole-sulfonamide hybrids along with their in vivo studies as anti-inflammatory agents on rats.
Solvents and chemicals were utilized without purification after obtaining from chemical suppliers. Thin layer chomatography (TLC) (Silica gel 60 F254 plates purchased from MERCK) were employed to monitor reaction and color less spot on TLC were analyzed under UV lamp, of short and long wavelength. All synthesized hybrids were purified by using silica gel column chomatography. Agilent Cary 630 FT-IRspectrometer were used to record IR spectra’s while 1H-NMR spectra were recorded on Bruker Avance 300 and 400 MHz spectrometer.
Synthesis of 1, 2, 5-oxadiazole-sulfonamide hybrids
The precursor 3, 4-diphenyl-1, 2, 5-oxadiazole used for the synthesized of all new sulfonamide hybrids were prepared by previous reporting method (Mange et al., 2007). 3, 4-Diphenyl-1, 2, 5-oxadiazole (1.0 mmol) (3) was taken in a round bottom flask (25 ml) and chlorosulfonic acid (6.0 mmol) was added drop wise at 0 oC with continuous stirring and allowed to attain room temperature after 10 minutues. After 17 h the reaction quenched with crushed ice and product extracted by using EtOAc (3 × 15 mL). The combined organic phase was washed with water and dried using anhydrous Na2SO4. To the dried organic layer, amine (1.0 mmol) was introduced drop wise with maintained temperature at 0 oC and reaction mixture further stirred for 2 h at room temperature. After 2 h reaction quenched by addition of water (10 mL). Then EtOAc (10 mL) was used thice for extraction and combine organic layer washed with water (10 mL) and dried using anhydrous Na2SO4. Solvent was removed under reduced pressure to obtain the crude residue of corresponding sulfonamide 4. Further purification of the resulting compound was achieved though column chomatography on silica gel using mixture of ethyl acetate and hexanes as an eluent.
Characterization data of synthesized compounds
3-[3-(Dimethylaminosulfonyl) phenyl]-4-phenyl-1,2,5-oxadiazole (4a)
Yield: 65% (213 mg), white solid; FT-IR (v-cm-1): 3020 (CHarom), 2923 (CHaliph,), 2870, 1455 (NO), 1341 (S-O, asym), 1162 (S-O, sym); 1H NMR (CDCl3, 400 MHz) δ 7.90–7.88 (m, 2H, H-2 and H-4), 7.79 (d, J = 7.6 Hz, 1H, H-6), 7.64–7.60 (m, 1H, H-5), 7.50–7.48 (m, 3H, H-2’, H-4’ and H-6’), 7.45–7.41 (m, 2H, H-3’ and H-5’), 2.65 (s, 6H, CH3); Anal. Calcd. for C16H15N3O3S: C, 58.34; H, 4.59; N, 12.76%. Found C, 58.19; H, 4.46; N, 12.63%.
3-{3-[Di(2-hydroxyethyl) aminosulfonyl] phenyl}-4-phenyl-1,2,5-oxadiazole (4b)
Yield: 60% (226 mg), white solid; FT-IR (v-cm-1): 3291 (OH str),3069 (CHarom), 2975 (CHaliph), 1588 (C=N), 1449 (NO), 1339 (S-O, asym), 1158 (S-O, sym); 1H NMR (CDCl3, 400 MHz) δ 7.95–7.93 (m, 2H, H-2 and H-4), 7.80 (d, J = 7.6 Hz, 1H, H-6),7.61 (t, J = 8.0 Hz, 1H, H-5), 7.5–7.43 (m, 5H, H-2’, H-3’, H-4’, H-5’ and H-6’), 3.81 (t, J = 4.8 Hz, 4H, O-CH2),3.20 (t, J = 4.8 Hz, 4H, N-CH2); Anal. Calcd. for C18H21N3O5S: C, 55.23; H, 5.41; N, 10.73%. Found C, 55.08; H, 5.50; N, 10.80%.
3-[3-(Propylaminosulfonyl) phenyl]-4-phenyl-1,2,5-oxadiazole (4c)
Yield: 57% (195 mg), white solid; FT-IR (v-cm-1)3267 (N-H, Sym), 3070 (CHarom), 2950 (CHaliph), 2875, 1592 (C=N), 1480 (NO), 1328 (S-O, asym), 1164 (S-O, sym);1H NMR (CDCl3, 300 MHz)δ 8.03(s, 1H, H-2), 7.97 (d, J = 7.8 Hz, 1H, H-4),7.71 (d, J = 7.8 Hz, 1H, H-6),7.57 (t, J = 7.8 Hz, 1H, H-5), 7.51–7.40 (m, 5H, H-2’, H-3’, H-4’, H-5’ and H-6’), 4.48 (s, 1H, N-H), 2.90–2.84 (m, 2H, N-CH2), 1.59–1.43 (m, 2H, CH2), 0.85 (t, J = 7.5 Hz, 3H, CH3); Anal. Calcd. for C17H17N3O3S: C, 59.46; H, 4.99; N, 12.24%. Found C, 59.61; H, 5.15; N, 12.34%.
3-[3-(phenylaminosulfonyl) phenyl]-4-phenyl-1, 2, 5-oxadiazole (4d)
Yield: 62% (233 mg), white solid; FT-IR (v-cm-1)3271 (N-H, Sym), 3066 (CHarom), 1601 (C=N), 1479 (NO), 1339 (S-O, asym), 1185 (S-O, sym); 1H NMR (CDCl3, 400 MHz)δ 8.00 (s, 1H, H-2), 7.83 (d, J = 8.0 Hz, 1H, H-4),7.67 (d, J = 7.6 Hz, 1H, H-6), 7.52–7.46 (m, 2H, H-2’ and H-6’), 7.43–7.38 (m, 4H, H-5, H-3’, H-4’ and H-5’), 7.25–7.21 (m, 2H, H-3’’ and H-5’’), 7.14–7.10 (m, 1H, H-4’’), 7.00 (d, J = 8.0 Hz, 2H, H-2’’ and H-6’’), 6.45 (s, 1H, N-H); Anal. Calcd. for C20H15N3O3S: C, 63.65; H, 4.01; N, 11.13%. Found C, 63.52; H, 3.89; N, 11.02%.
3-{3-[(4-Methylphenyl) aminosulfonyl] phenyl}-4-phenyl-1, 2, 5-oxadiazole (4e)
Yield: 64% (250 mg), white solid; FT-IR (v-cm-1) 3257 (N-H, sym), 3067 (CHarom), 2922 (CHaliph), 1447 (NO), 1365 (S-O, asym), 1160 (S-O, sym); 1H NMR (CDCl3, 400 MHz)δ 7.96 (s, 1H, H-2), 7.80 (d, J = 8.0 Hz, 1H, H-4),7.66 (d, J = 8.0 Hz, 1H, H-6), 7.50–7.48 (m, 2H, H-2’ and H-6’), 7.43–7.40 (m, 4H, H-5, H-3’, H-4’ and H-5’), 7.02 (d, J = 8.0 Hz, 2H, H-3’’ and H-5’’), 6.88 (d, J = 8.0 Hz, 2H, H-2’’ and H-6’’), 6.26 (s, 1H, N-H), 2.26 (s, 3H, CH3); Anal. Calcd. for C21H17N3O3S: C, 64.43; H, 4.38; N, 10.73%. Found C, 64.31; H, 4.26; N, 10.64%.
3-{3-[(3-Methylphenyl) aminosulfonyl] phenyl}-1, 2, 5-oxadiazole (4f)
Yield: 60% (234 mg), white solid; FT-IR (v-cm-1) 3254 (N-H, sym), 3065 (CHarom), 2940 (CHaliph), 1594 (C=N), 1459 (NO), 1326 (S-O, asym), 1160 (S-O, sym); 1H NMR (CDCl3, 400 MHz)δ 8.00 (s, 1H, H-2), 7.84 (d, J = 8.0 Hz, 1H, H-4),7.66 (d, J = 7.6 Hz, 1H, H-6), 7.51–7.46 (m, 2H, H-2’ and H-6’), 7.44–7.38 (m, 4H, H-5, H-3’, H-4’ and H-5’), 7.12–7.08 (m, 1H, H-5’’), 6.93 (d, J = 7.6 Hz, 1H, H-4’’),6.84 (s, 1H, H-2’’), 6.79 (d, J = 8.0 Hz, 1H, H-6’’), 6.34 (s, 1H, N-H), 2.26 (s, 3H, CH3); Anal. Calcd. for C21H17N3O3S: C, 64.43; H, 4.38; N, 10.73%. Found C, 64.28; H, 4.25; N, 10.61%.
3-{3-[(4-Hydroxyphenyl) aminosulfonyl] phenyl}-1,2,5-oxadiazole (4g)
Yield: 65% (255 mg), white solid; FT-IR (v-cm-1) : 3392 (OH str), 3260 (NH str), 3061 (CHarom), 1602 (C=N), 1449 (NO), 1326 (S-O, asym), 1154 (S-O, sym); 1H NMR (DMSO–d6, 300 MHz)δ 9.83 (s, 1H, O-H), 9.32 (s, 1H, N-H), 7.86 (s, 1H, H-2), 7.81–7.78 (m, 1H, H-4), 7.69–7.64 (m, 2H, H-5 and H-6 ), 7.57–7.54 (m, 1H, H-4’), 7.50–7.43 (m, 4H, H-2’, H-3’, H-5’ and H-6’), 6.78 (d, J = 8.7 Hz, 2H, H-3’’ and H-5’’), 6.58 (d, J = 9.0 Hz, 2H, H-2’’ and H-6’’); Anal. Calcd. for C20H15N3O4S: C, 61.06; H, 3.84; N, 10.68%. Found C, 60.93; H, 3.69; N, 10.55%.
3-{3-[(2-Hydroxyphenyl) aminosulfonyl] phenyl}-1, 2, 5-oxadiazole (4h)
Yield: 62% (243 mg), white solid; FT-IR (v-cm-1): 3389 (OH str), 3272 (NH str), 3061 (CHarom), 1598 (C=N), 1447 (NO), 1336 (S-O, asym), 1156 (S-O, sym); 1H NMR (CDCl3, 400 MHz)δ 7.99 (s, 1H, H-2), 7.80 (d, J = 7.6 Hz, 1H, H-4),7.68 (d, J = 8.0 Hz, 1H, H-6), 7.52–7.46 (m, 3H, H-5, H-2’ and H-6’), 7.44–7.42 (m, 3H, H-3’ H-4’ and H-5’), 7.08–7.04 (m, 1H, H-4’’), 6.91–6.89 (m, 1H, H-6’’), 6.85–6.82 (m, 1H, H-3’’), 6.75 (t, J = 7.6 Hz, 1H, H-5’’), 6.43 (s, 1H, N-H); Anal. Calcd. for C20H15N3O4S: C, 61.06; H, 3.84; N, 10.68%. Found C, 61.24; H, 3.65; N, 10.79%.
3-{3-[(2,4-Dimethylphenyl) aminosulfonyl] phenyl}-1,2,5-oxadiazole (4i)
Yield: 63% (255 mg), white solid; FT-IR (v-cm-1) 3264 (N-H, sym), 3068 (CHarom), 2928 (CHaliph), 1594 (C=N), 1446 (NO), 1330 (S-O, asym), 1160 (S-O, sym); 1H NMR (CDCl3, 400 MHz) δ 7.96–7.96 (m, 1H, H-2), 7.79–7.76 (m, 1H, H-4), 7.69–7.67 (m, 1H, H-6), 7.50–7.47 (m, 2H, H-2’ and H-6’), 7.46–7.40 (m, 4H, H-5, H-3’, H-4’ and H-5’), 7.02 (d, J = 8.8 Hz, 1H, H-5’’), 6.89 (m, 2H, H-3’’ and H-6’’), 6.10 (s, 1H, N-H); Anal. Calcd. for C22H19N3O3S: C, 65.17; H, 4.72; N, 10.36%. Found C, 65.01; H, 4.79; N, 10.22%.
3-{3-[(3,4-Dimethylphenyl) aminosulfonyl] phenyl}-1,2,5-oxadiazole (4j)
Yield: 67% (271 mg), white solid; FT-IR (v-cm-1) 3253 (N-H, sym), 3064 (CHarom), 2923 (CHaliph), 1488 (NO), 1361 (S-O, asym), 1161 (S-O, sym); 1H NMR (CDCl3, 400 MHz)δ 7.98 (s, 1H, H-2), 7.81 (d, J = 8.0 Hz, 1H, H-4),7.65 (d, J = 7.6 Hz, 1H, H-6), 7.51–7.45 (m, 2H, H-2’ and H-6’), 7.44–7.38 (m, 4H, H-5, H-3’, H-4’ and H-5’ ), 6.96 (d, J = 8.0 Hz, 1H, H-5’’), 6.79 (s, 1H, H-2’’), 6.72–6.70 (m, 1H, H-6’’), 6.27 (s, 1H, N-H),2.16–2.15 (m, 6H, CH3); Anal. Calcd. for C22H19N3O3S: C, 65.17; H, 4.72; N, 10.36%. Found C, 65.04; H, 4.81; N, 10.44%.
Experimental animals
Wistar albino rats of either sex weighing 150-180 g were used during the anti-inflammation activity. The rats were kept in animal house under standard condition and provided free access of laboratory standard food and water. Further the care of rats was carried out by following the international ethical guidelines. Animal experimental were approved by institutional ethical committee and adequate consideration were adopted during all tests to minimize discomfort of rats as well as reduce pain (El-Miedany et al., 2006).
In vivo anti-inflammatory activity
The anti-inflammatory potential of newly synthesized compounds (4a-4j) was evaluated in vivo by using carrageenan induced rat paw edema model reported by winter et al. with slight modification (Winter et al., 1962). Celecoxib and indomethacin were used as a standard reference drugs in this study and the results of test compounds were compared with them. Solutions of test compounds, celecoxib and indomethacin in 10 % tween-80 were administered orally in separated groups at a dose of 10 mg/Kg. While the control group was received 1 mL saline only. After one hour, edema was induced though 0.1 mL of carrageenan solution (1%) injection to the right hind paw of the rats in all groups. The volume of rat’s paw was measured by using plethysmometer immediately after carrageenan injection and then with the interval of one hour four times. The following equation were employed to measure the percentage of edema.
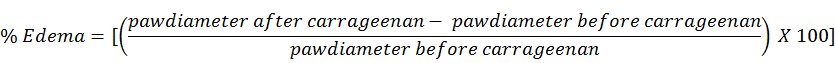
RESULTS AND DISCUSSION
1, 2, 5-oxadiazole-sulfonamide hybrids
The synthesis routes of the target 1, 2, 5-oxadiazole-sulfonamide hybrids (4a-4j) is outlined in Scheme 1. First, key intermediate 3, 4-diphenyl-1, 2, 5-oxadiazole (3) was synthesized from benzyl (1) and hydroxyl amine followed by dehydration according to reported method. Mange et al. (2007) Chlorosulfonation of 3 and subsequent condensation with appropriate aliphatic or aromatic amine afforded the desired 3-[3-(alkyl/aryl aminosulfonyl) phenyl]-4-phenyl-1, 2, 5-oxadiazole (4a-4j) in moderate to good yield. Chemical structures of all the synthesized hybrids (4a-4j) were verified by using spectroscopic techniques including, FT-IR, 1H-NMR spectroscopy.
In the FT-IR spectrum, primary and secondary sulfonamides were distinguished by the presence or absence of N-H stretching band between 3250-3275 cm-1 respectively. While the presence of hydroxyl group confirmed by O-H stretching band around 3390 and 3290 cm-1 in aromatic and aliphatic sulfonamides, respectively. The bands appeared slightly above and slightly below 3000 cm-1 attributed the C-H stretching of aromatic and aliphatic systems, respectively. The C=N bond appeared in range of 1588-1602 cm-1 and the most prominent bands around 1340 and 1160 cm-1 confirmed the presence of SO2 group. Further proton NMR spectra of 1,2,5-oxadiazole-sulfonamide hybrids also confirmed their complete structures of synthesized compounds. The peaks of all protons have assigned in experimental section. In 1H-NMR spectra, a signal (usually singlet) around δ 8.00 ppm corresponding to the isolated proton on the substituted phenyl ring was observed in each case rather than a pattern due to, for example, a para substitution. This outcome of the reaction is somewhat surprising. The most reactive positions in the molecule for electrophilic substitution would be expected to be para-substitution of the phenyl ring. Even Vela´zquez et al. (2005) have reported the formation of 3-[4-(aminosulfonyl) phenyl]-4-phenyl-1, 2, 5-oxadiazole from 3, 4-diphenyl-1, 2, 5-oxadiazole in a two-step reaction involving chlorosulfonation with chlorosulfonic acid followed by reaction with ammonium hydroxide (30%) (Velázquez et al., 2005). Probably they would have isolated the only that isomer (might not be major product) which has been prepared by an alternative route involving the N-oxide of the 3, 4-diphenyl-1, 2, 5-oxadiazole. The outcome of meta-substitution can be explained on the basis that prior to chlorosulfonation, protonation occurred at least at one of the nitrogen atoms of the heterocyclic ring which lead to a deactivation of the central 1, 2, 5-oxadiazole moiety and at the same time favoring meta- substitution due to electron withdrawal from the phenyl groups (Fig. 2).
Anti-inflammatory activity
All the synthesized 1, 2, 5-oxadiazole-sulfonamide hybrids (4a-4j) were screened in-vivo for their anti-inflammatory potential though carrageenan-induced rat paw edema method using standard drugs celecoxib and indomethacin as a reference. Delightfully all the tested compounds exhibited moderate to excellent anti-inflammatory activity in comparison to the reference drugs. The results of these studies are expressed as % edemaat a dose of 10 mg/Kg at different time intervals with one-hour gape and presented in Table I.
Table I. Anti-inflammatory results of 1, 2, 5-oxadiazole-sulfonamide hybrids (4a-4j).
|
Compounds code |
Zero h |
1 h |
2 h |
3 h |
4 h |
||||
|
PD |
PD |
% Edema |
PD |
% Edema |
PD |
% Edema |
PD |
% Edema |
|
|
Control |
3.47±0.18 |
4.53±0.20 |
30.54 |
4.75±0.18 |
36.88 |
4.83±0.15 |
39.19 |
4.91±0.16 |
41.49 |
|
4a |
3.50±0.10 |
4.10±0.12 |
17.14 |
4.15±0.08 |
18.57 |
4.02±0.13 |
14.85 |
3.96±0.12 |
13.14 |
|
4b |
3.47±0.08 |
3.87±0.09 |
11.52 |
3.81±0.08 |
9.79 |
3.75±0.10 |
8.06 |
3.70±0.08 |
6.62 |
|
4c |
3.50±0.17 |
4.25±0.12 |
21.42 |
4.16±0.18 |
18.85 |
4.11±0.15 |
17.42 |
4.04±0.12 |
15.42 |
|
4d |
3.48±0.12 |
4.02±0.09 |
15.51 |
4.05±0.13 |
16.37 |
3.93±0.11 |
12.93 |
3.90±0.15 |
12.06 |
|
4e |
3.52±0.13 |
3.93±0.13 |
11.64 |
3.88±0.14 |
10.22 |
3.84±0.12 |
9.09 |
3.78±0.10 |
7.38 |
|
4f |
3.54±0.15 |
3.95±0.16 |
11.58 |
3.90±0.18 |
10.16 |
3.89±0.17 |
9.88 |
3.85±0.17 |
8.75 |
|
4g |
3.49±0.25 |
3.89±0.21 |
11.46 |
3.82±0.19 |
9.45 |
3.77±0.18 |
8.02 |
3.71±0.20 |
6.30 |
|
4h |
3.53±0.14 |
3.91±0.15 |
10.76 |
3.85±0.14 |
9.06 |
3.84±0.16 |
8.78 |
3.78±0.13 |
7.08 |
|
4i |
3.51±0.09 |
4.12±0.07 |
17.37 |
4.01±0.08 |
14.24 |
3.94±0.10 |
12.25 |
3.91±0.10 |
11.39 |
|
4j |
3.53±0.22 |
4.21±0.20 |
19.26 |
4.11±0.18 |
16.43 |
4.07±0.20 |
15.29 |
3.98±0.22 |
12.74 |
|
Celecoxib |
3.51±0.16 |
3.92±0.20 |
11.68 |
3.86±0.12 |
9.97 |
3.82±0.12 |
8.83 |
3.76±0.14 |
7.12 |
|
Indomethacin |
3.52±0.13 |
3.92±0.15 |
11.36 |
3.87±0.16 |
9.94 |
3.83±0.15 |
8.80 |
3.77±0.14 |
7.10 |
Abbreviation: PD, paw diameter (mm). Data are expressed as mean ± SE. All values had p < 0.05 (SPSS software).
Structure-activity relationship (SAR) investigations for all the synthesized hybrids was established on the bases of nature of amine and the position of its substituents. Among all derivatives, compound 4g having hydroxyl group at pera-position of aniline was found the most potent with 6.30 % edema after four h as compare to both reference drugs celecoxib and indomethacin that showed 7.12 and 7.10% after the same time. Compound 4h, having hydroxyl group at ortho-position of aniline, showed less potency than 4g but equivalent to reference drugs. While aniline and it’s all other mono and disubstituted derivatives showed comparable and less potency as compare to standard drugs. Further, mono-substituted derivatives especially pera-substituted showed better results as compare to disubstituted derivatives. Among thee aliphatic amines only 4b showed better results than reference drugs while the other two aliphatic amine derived compounds (4a and 4c) show moderate activity. Based on above mentioned results, it can be easily observed the presence of hydroxyl group significantly enhance the anti-inflammatory activity of these compounds.
CONCLUSION
In the present study, we designed and synthesized a novel series of 1,2,5-oxadiazole-sulfonamide hybrids (4a-4j) in search of potent anti-inflammatory agent. All synthesized hybrids were characterized by spectroscopic techniques and screened for in vivo anti-inflammatory activities by using carrageenan-induced paw edema method. All compounds exhibited moderate to excellent anti-inflammatory potential. Among all, two analogs 4b and 4g showed more potency as compared to standard drugs celecoxib and indomethacin. Delightfully, thee analogs 4c,4g and 4k also exhibited comparable anti-inflammatory activity with reference compounds. Hopefully, these compounds may serve in future investigations as potential candidates for the development of more potent anti-inflammatory agent after their molecular level investigation.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Batool, M., Tajammal, A., Farhat, F., Verpoort, F., Khattak, Z.A.K., Meh un, N., Shahid, M., Ahmad, H.A., Munawar, M.A., Zia-ur-Rehman, M., and Basra, M.A.R., 2018. Molecular docking, computational, and antithombotic studies of novel 1,3,4-oxadiazole derivatives. Int. J. mol. Sci., 19: 3606/3601-3606/3618. https://doi.org/10.3390/ijms19113606
Burnier, M., 2005. The safety of rofecoxib. Expert Opin. Drug Safe., 4: 491-499. https://doi.org/10.1517/14740338.4.3.491
Caro, V.D., Sutera, F.M., and Giannola, L.I., 2017. In situ delivery of corticosteroids for treatment of oral diseases. Ther. Deliv., 8: 899-914. https://doi.org/10.4155/tde-2017-0055
Carullo, G., Galligano, F., and Aiello, F., 2017. Structure activity relationships for the synthesis of selective cyclooxygenase 2 inhibitors: An overview (2009–2016). Med. Chem. Commun., 8: 492-500. https://doi.org/10.1039/C6MD00569A
Chan, C.C., Boyce, S., Brideau, C., Charleson, S., Cromlish, W., Ethier, D., Evans, J., Ford-Hutchinson, A.W., Forrest, M.J., Gauthier, J.Y., Gordon, R., Gresser, M., Guay, J., Kargman, S., Kennedy, B., Leblanc, Y., Leger, S., Mancini, J., O’Neill, G.P., Ouellet, M., Patrick, D., Percival, M.D., Perrier, H., Prasit, P., Rodger, I., Tagari, P., Therien, M., Vickers, P., Visco, D., Wang, Z., Webb, J., Wong, E., Xu, L.J., Young, R.N., Zamboni, R., and Riendeau, D., 1999. Rofecoxib [Vioxx, MK-0966; 4-(4’-methylsulfonylphenyl)-3-phenyl-2-(5H)-furanone]: a potent and orally active cyclooxygenase-2 inhibitor. Pharmacological and biochemical profiles. J. Pharmacol. exp. Ther., 290: 551-560.
Chaudhy, F., Naureen, S., Ashaf, M., Al-Rashida, M., Jahan, B., Munawar, M.A., and Khan, M.A., 2019. Imidazole-pyrazole hybrids: Synthesis, characterization and in-vitro bioevaluation against α-glucosidase enzyme with molecular docking studies. Bioorg. Chem., 82: 267-273. https://doi.org/10.1016/j.bioorg.2018.10.047
Dhingra, A.K., Chopra, B., Dua, J.S., and Parsad, D.N., 2017. New insight on inflammation and its management: A review. J. Innov. Pharm. Biol. Sci., 4: 117-126.
El-Miedany, Y., Youssef, S., Ahmed, I., and El-Gaafary, M., 2006. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox-2 inhibitor in inflammatory bowel diseases. Am. J. Gastroenterol., 101: 311-317. https://doi.org/10.1111/j.1572-0241.2006.00384.x
Fabiola, G.F., Damodharan, L., Pattabhi, V., and Nagarajan, K., 2001. Cyclooxygenase-2. An attractive target for fruitful drug design. Curr. Sci., 80: 26-34.
Fanelli, A., Ghisi, D., Aprile, P.L., and Lapi, F., 2017. Cardiovascular and cerebrovascular risk with nonsteroidal anti-inflammatory drugs and cyclooxygenase 2 inhibitors: latest evidence and clinical implications. Ther. Adv. Drug Safe., 8: 173-182. https://doi.org/10.1177/2042098617690485
Fauzia, A.C., Samina, K., Meh, N., and Munawar, A.M., 2019. Synthesis of 3-Arylindazole-1-acetic Acids and In Vitro Study of Potential Antibacterial Effect. Lett. Drug Design Discov., 16: 401-407. https://doi.org/10.2174/1570180815666180702152259
Grivennikov, S.I., Greten, F.R., and Karin, M., 2010. Immunity, inflammation, and cancer. Cell (Cambridge, MA, U.S.), 140: 883-899. https://doi.org/10.1016/j.cell.2010.01.025
Hodge, G., and Hodge, S., 2019. Therapeutic targeting steroid resistant pro-inflammatory NK and NKT-Like cells in chonic inflammatory lung disease. Int. J. mol. Sci., 20: 1511. https://doi.org/10.3390/ijms20061511
Mange, Y., Shikant, S., Devendra, P., Pinkal, P., Hetal, P., Arvind, P., Balaraman, R., and Rajani, G., 2007. Studies in 3, 4-diaryl-1, 2, 5-oxadiazoles and their N-oxides: Search for better COX-2 inhibitors. Acta Pharm., 57: 13-30. https://doi.org/10.2478/v10007-007-0002-z
Marzano, A.V., Ortega-Loayza, A.G., Heath, M., Morse, D., Genovese, G., and Cugno, M., 2019. Mechanisms of inflammation in neutrophil-mediated skin diseases. Front. Immunol., 10: 1059. https://doi.org/10.3389/fimmu.2019.01059
Medzhitov, R., 2008. Origin and physiological roles of inflammation. Nature (London, U.K.), 454: 428-435. https://doi.org/10.1038/nature07201
Medzhitov, R., 2010. Inflammation 2010: new adventures of an old flame. Cell (Cambridge, MA, U.S.), 140: 771-776. https://doi.org/10.1016/j.cell.2010.03.006
Nasef, N.A., Mehta, S., and Ferguson, L.R., 2017. Susceptibility to chonic inflammation: An update. Arch. Toxicol., 91: 1131-1141. https://doi.org/10.1007/s00204-016-1914-5
Padi, S.S.V., Jain, N.K., Singh, S., and Kulkarni, S.K., 2004. Pharmacological profile of parecoxib: A novel, potent injectable selective cyclooxygenase-2 inhibitor. Eur. J. Pharmacol., 491: 69-76. https://doi.org/10.1016/j.ejphar.2004.03.013
Papanagnou, P., Baltopoulos, P., and Tsironi, M., 2015. Marketed nonsteroidal anti-inflammatory agents, antihypertensives, and human immunodeficiency virus protease inhibitors: as-yet-unused weapons of the oncologists’ arsenal. Ther. Clin. Risk Manage., 11: 807-819. https://doi.org/10.2147/TCRM.S82049
Praveen, R.P.N., and Knaus, E.E., 2008. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J. Pharm. Pharm. Sci., 11: 81s-110s. https://doi.org/10.18433/J3T886
Radi, Z.A., and Khan, K.N., 2019. Cardio-renal safety of non-steroidal anti-inflammatory drugs. J. toxicol. Sci., 44: 373-391. https://doi.org/10.2131/jts.44.373
Ricciotti, E., and FitzGerald, G.A., 2011. Prostaglandins and Inflammation. Arterioscler. Thomb. Vasc. Biol., 31: 986-1000. https://doi.org/10.1161/ATVBAHA.110.207449
Riendeau, D., Percival, M.D., Brideau, C., Charleson, S., Dube, D., Ethier, D., Falgueyret, J.P., Friesen, R.W., Gordon, R., Greig, G., Guay, J., Mancini, J., Ouellet, M., Wong, E., Xu, L., Boyce, S., Visco, D., Girard, Y., Prasit, P., Zamboni, R., Rodger, I.W., Gresser, M., Ford-Hutchinson, A.W., Young, R.N., and Chan, C.C., 2001. Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J. Pharmacol. exp. Ther., 296: 558-566.
Scheiman, J.M., 2016. NSAID-induced gastrointestinal injury: a focused update for clinicians. J. Clin. Gastroenterol., 50: 5-10. https://doi.org/10.1097/MCG.0000000000000432
Sehajpal, S., Prasad, D.N., and Singh, R.K., 2018. Prodrugs of non-steroidal anti-inflammatory drugs (NSAIDs): A long march towards synthesis of safer NSAIDs. Mini-Rev. Med. Chem., 18: 1199-1219. https://doi.org/10.2174/1389557518666180330112416
Silverstein, F.E., Faich, G., Goldstein, J.L., Simon, L.S., Pincus, T., Whelton, A., Makuch, R., Eisen, G., Agrawal, N.M., Stenson, W.F., Burr, A.M., Zhao, W.W., Kent, J.D., Lefkowith, J.B., Verburg, K.M., and Geis, G.S., 2000. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthitis and rheumatoid arthitis. The CLASS study: a randomized controlled trial. J. Am. med. Assoc., 284: 1247-1255. https://doi.org/10.1001/jama.284.10.1247
Thomas, D., Ali, Z., Zachariah, S., Sundararaj, K.G.S., Van Cuyk, M., and Cooper, J.C., 2017. Coxibs refocus attention on the cardiovascular risks of non-aspirin NSAIDs. Am. J. Cardiovasc. Drugs, 17: 343-346. https://doi.org/10.1007/s40256-017-0223-6
Velázquez, C., Rao, P.N.P., McDonald, R., and Knaus, E.E., 2005. Synthesis and biological evaluation of 3, 4-diphenyl-1, 2, 5-oxadiazole-2-oxides and 3,4-diphenyl-1,2,5-oxadiazoles as potential hybrid COX-2 inhibitor/nitric oxide donor agents. Bioorg. med. Chem., 13: 2749-2757. https://doi.org/10.1016/j.bmc.2005.02.034
Winter, C.A., Risley, E.A., and Nuss, G.W., 1962. Carrageenin-induced edema in hind paw of rat as an assay for antiinflammatory drugs. Proc. Soc. exp. Biol. Med., 111: 544-547. https://doi.org/10.3181/00379727-111-27849
To share on other social networks, click on any share button. What are these?









