Genotypic Screening of Maize (Zea mays L.) for Salt Tolerance at Early Growth Stage under Different Salinity Levels
Genotypic Screening of Maize (Zea mays L.) for Salt Tolerance at Early Growth Stage under Different Salinity Levels
Shamsher Ali1*, Muhammad Jamal Khan2, Zahir Shah2, Naveedullah3 and Abdul Jalal1
1Department of Soil and Environmental Sciences, Amir Muhammad Khan Campus Mardan, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 2Department of Soil and Environmental Sciences, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 3Department of Water Management, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | Soluble salts and exchangeable sodium in soils are the main constraints in the agriculture ecosystem creating major threats to crop productivity and, hence, affecting socio economic condition of the farming community. Growing of salt tolerant crop genotypes of species on salt affected lands is an effective tool for getting desirable productivity. A two-factor experiment using complete randomized design with three replications in pots having silt loam soil was carried out on April 03, 2016 to examine three maize genotypes (Iqbal, Jalal, and Azam) under different salinity levels i.e. 0.4, 2.7, 5.2 and 7.6 dS m-1 at Amir Muhammad Khan Campus, Mardan, the University of Agriculture, Peshawar. The analyzed data parameters were percent emergence, plant height, number of leaves, shoots and roots weight. The results revealed that all data parameters except number of leaves were affected significantly (p < 0.05) by different levels of salinity. Irrespective of maize varieties, the percent emergence, plant height, number of leaves, shoots and roots weight of Maize were reduced with linear increasing levels of salinity. Among the genotypes, maximum 1st, 2nd percent emergence (50% and 96 % respectively), plant height (94 cm), number of leaves (10), shoot weight (59 g/pot) and root weight (9 g/pot) were recorded by Iqbal genotypes followed by Jalal genotypes irrespective of various salinity levels. The results also showed that Iqbal genotype performed better at each salinity level and is concluded to be grown and extended on salt affected areas of the District Mardan as well as in central Khyber Pakhtunkhwa.
Received | January 03, 2018; Accepted | January 22, 2019; Published | February 19, 2019
*Correspondence | Shamsher Ali, Department of Soil and Environmental Sciences, Amir Muhammad Khan Campus Mardan, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: shamsherali@aup.edu.pk
Citation | Ali, S., M.J. Khan, Z. Shah, Naveedullah and A. Jalal. 2019. Genotypic screening of maize (Zea mays L.) for salt tolerance at early growth stage under different salinity levels. Sarhad Journal of Agriculture, 35(1): 208-215.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.1.208.215
Keywords | Screening, Maize genotypes, Emergence, Morphological parameters, Induced salinity levels
Introduction
Maize (Zea mays L.) belongs to the grass family of Poaceae and is cross pollinated. Its first growth was started by the people in ancient Central America and expanded to other parts of the world. Globally, maize has third position in rank among the cereals. Being angiosperm, its seeds are enclosed in a shell. In developing world, most of the people use it as their staple and principal food while in advance countries, it is consumed as an animal feed and other byproducts. Due to its higher yielding nature, maize is a key crop for countries with higher/more population like Pakistan to meet their food requirements.
In Pakistan, maize is on 3rd important position in the cereal crops after wheat and rice, while in province Khyber Pakhtunkhwa (KPK), ranked 2nd cereal crop after wheat and is grown in irrigated and non- irrigated areas mainly in northern belt of KPK including district Mardan. The lands of central KPK including district Mardan, Charsadda, Nowshehra, Swabi and Peshawar are affected by various kind of Salts being maize growing belt. The relative salt tolerance limits for maize crop are ECe at 2.5, 4.0 and 6.0 dS m-1 corresponding to 10 %, 25% and 50% reduction in yield respectively. About 6.10 million hectares (m ha) land is affected in Pakistan by various kind of salts. Substantial yield losses and hence crop productivity is caused by salinity (Cha-um et al., 2011). In dry areas of the earth, on one hand where the annul precipitation in any form is low than evapotranspiration and on other hand the faulty management of soil and water, increased the salinity affecting the crop productivity (Munns, 2002). In the world, about 831 m ha lands are salt affected, out of which saline is 398 m ha and 433 m ha is sodic (FAO, 2005; Munns, 2005). Various changes in plant metabolism like inhibition of enzymes activity, variation in phosphorylation state and oxidative damages of cells in maize are caused by excess salts (De Azevedo Neto et al., 2006). Soils contain soluble salt with electrical conductivity (EC) value ≥ 4 dS m-1 or 40 mM salts are saline soils which directly affect the emergence, growth and yield of various crop species (Boyer, 1982; Allakhverdiev et al., 2000). Some of the vegetable and even crops have shown sensitivity to the salinity range of 1.0 to 1.8 dS m-1 by reducing the productivity with a range of 5-20% while some of the cereal crops like barley, wheat, rice and maize etc. can tolerate and grow under the range of 5-18 dS m-1 salinity. Also, the various species of the same genera can tolerate various salinity levels.
The biological method of reclamation is growing of salt tolerant crops and species, which is easier, less costly and manageable which include screening, selection through conformist breeding, assessment and development of plants for salt tolerance, use of growth regulators and antioxidants as well as microbial application.
Different maize varieties are grown in KPK. All of them have their own characteristics in terms of yield, cultivation and production tested in non-saline soils. Limited or no work has been done on the response of different maize genotypes in saline soils of KPK. Maize is C4 plant and considered as moderately sensitive crop to salts contents (Maas et al., 1983; Chinnusamy et al., 2005). The extensive specific genomic difference for salt tolerance occurs in maize (Sharif et al., 1999; Mansour et al., 2005; khan et al., 2003; Zahoor et al., 2011).
Keeping in view the importance of maize in the economy of Pakistan and above constrains, a pot experiment was carried out to examine the performance of maize varieties under various salinity concentrations i.e. 0.4, 2.7, 5.2 and 7.6 dS m-1 and then the screened salt tolerant material/genotype of maize will be grown by the farmers in the salt affected rural areas.
Materials and Methods
A research trial in pots was carried out in rain protected net/wire house of natural environment at Amir Muhammad Khan Campus, Mardan of the University of Agriculture, Peshawar-Pakistan during 2016 to evaluate three locally developed maize genotypes under four different induced salts stress conditions i.e. 0.4 (original), 2.7, 5.2, 7.6 dS m-1. The experiment was conducted in completely randomized design (CRD) with two factors; i) salinity levels ii) maize genotypes and repeated three times. The maize genotypes were Iqbal, Jalal, and Azam received from Cereal Crops Research Institute (CCRI), Pir Sabak, Nowshera, Khyber Pakhtunkhwa of Pakistan.
Pot experiment
The soil used for the experiment was collected from a depth of 0-18 cm from a farmer’s field, located near Amir Muhammad Khan Campus, Mardan, Khyber Pakhtunkhwa province of Pakistan. Before filling the pots, the soil sample was taken, processed and analyzed for some physico-chemical properties according to the standard methods and procedures as described by Ryan et al. (2013). Table 1 showed that the soil had electrical conductivity (EC1:2) of 0.4 dS m–1 and pH1:2 (7.63) means alkaline in reaction. Soil was moderately calcareous. According to laboratory tests, soil was also low in organic matter, AB-DTPA extractable P and K. The textural class of the soil was silt loam with a bulk density of 1.32 g cm-3 (Table 1).
A total number of 36 pots (diameter, 20 cm and height, 32 cm) made of plastic were lined and filled each with 4 kg soil. All the pots were labeled with four salinities levels 0.4, 2.7, 5.2, 7.6 dS m-1 for three varieties in three replications. The pots were arranged in wire house of the Department at ambient light and temperature according to CRD. The original EC of the soil was 0.4 dS m-1 and the other three levels were induced by the application of NaCl salt as required of EC= 2.7, 5.2 and 7.6 dS m-1 through the following procedures.
Table 1: Characterization of soil before sowing in pots.
| Soil Parameters | Values | Soil parameters | Values |
|
EC (1:2) dS m-1 |
0.4 | % Clay | 19 |
|
pH (1:2) |
7.63 | % Silt | 64 |
|
*AB-DTPA Ext. P ( mg kg-1) |
3.64 | % Sand | 17 |
|
AB-DTPA Ext. K (mg kg-1) |
112 | Textural Class | Silt loam |
|
% CaCO3 |
8.00 | % organic matter | 0.72 |
| Saturation percentage | 34 % | Bulk density |
1.32 g cm-3 |
*Ammonum Bicarbonate-Diethylene Triamine Pentacetic Acid Extractable.
Procedure and calculation for developing induced salinity
For this, we determined the original Electrical conductivity and saturation percentage of the soil which is 0.4 dS m-1 and 34 % respectively.
To develop the induced EC i.e 2.7, 5.2 and 7.6 dS m-1 is as under. First, in case of 2.7 dS m-1, the original EC of Soil was 0.4 dS m-1 and to develop the induced EC of 2.7 dS m-1.
Variations between two ECs = 2.7-0.4= 2.3 dS m-1, the salts of this EC (2.3 dS m-1) was applied according to the method of Hand Book 60 (Richards, 1954). A solution or soil having 1 dS m-1 containing 10 milli equivalents (m. eq.) of salts per Kg or 10 mM.
So 1 dS m-1 = 10 m.eq. salts
2.3 dS m-1 = 2.3 x 10 = 23 milli eq. kg-1 (Required milli eq.)
milli equivalent weight (m.eq.wt.) of NaCl is 58.5 mg and the saturation percentage of the soil is 34%.
Amount of NaCl for 2.3 dS m-1 to reach/develop the 2.7 dS m-1.
= Required milli eq. kg-1 x m. eq.wt. of NaCl
= 23 m.eq. kg -1 x 58.5 mg
= 1345.5 mg ÷1000 to convert in gram
= 1.3455 g kg-1
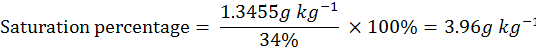
So, 3.96 g NaCl was required for one kg soil for the development of 2.7 dS m-1 EC and for 4 kg soil need NaCl as 3.96 x 4= 15.84 g NaCl for pots labeled with 2.7 dS m-1.
Similarly, the same procedure was adapted for the development of 5.2, 7.6 dS m-1 salinity.
In such a way, 33.03 g and 49.55 g NaCl was applied for the development of 5.2 and 7.6 dS m-1 induced salinity respectively.
Seven seed of maize (Zea mays L.) of each genotype was sown in each pot randomly on 3rd April 2016. Pots were irrigated with good quality tap water on saturation percentage basis whenever required. Recommended and calculated basal dose of N, P2O5, K2O fertilizers (120-100-60 kg ha-1) was applied as urea, single super phosphate and sulfate of potash respectively in each pot. Full dose of P and K along with 1/3rd of N was applied at sowing. The remaining N was applied at two split dose. Pots were rearranged and shuffled on daily basis randomly to get uniform environment. After emergence, in each pot only three seedlings were left for growth and growth parameters. Plants were grown for about six weeks after emergence and harvested on May 20, 2016. Data on some parameters were recorded on maize crop.
Data collected on the following parameters of maize
Emergence (%): Emergence was recorded at two intervals; first emergence was taken after four days of sowing and second after completion of emergence and then converted to % emergence at each stage.
Numbers of leaves: The number of leaves was counted from base to top of the three plants in each pot and was averaged.
Plant height (cm): In each pot the plant height of the three plants from base to top were measured and then averaged.
Shoot weight (g): After six week of maturity, the shoot of plants of each pot were harvested at the base, and dried and then weighted with the help of electric balance.
Root weight (g): After shoot harvesting, all the pots were flooded with water by losing the roots from soil then roots were dig out from mud of pots and washed with the help of water, air dried and then weighted on electronic balance.
Statistical analysis: Data on maize parameters were passed through a package of Statistix 8.1 using a two-factor CR design. The least significance difference test at probability of 0.05 was run to separate the means (Steel and Torrie, 1984)
Results and Discussion
Emergence was recorded at two Intervals
First percent emergence (emergence after four days of sowing): The data on percent (%) emergence (after four days of sowing) of maize were subjected to statistical analysis (Table 2) and revealed that % emergence of maize genotypes were affected considerably (p ≤ 0.05) by different salinity concentration/levels. The lowest emergence (18%) was recorded in 7.6 dS m-1, while the maximum (67%) in 0.4 dS m-1. As regard means of maize genotypes, the percent emergence (50 %) of Iqbal genotypes was highest significantly (P < 0.05), followed by Jalal (35 %) and lowest emergence (29 %) by Azam genotypes.
Table 2: First Percent emergence (after 4 days of sowing) of maize as affected by various induced salinity levels.
| Maize genotypes | Induced salinity levels (dS m-1) | Means of genotypes | |||
| 0.4 | 2.7 | 5.2 | 7.6 | ||
| Percent (%) emergence | |||||
| Iqbal | 86 | 57 | 29 | 28 | 50 a |
| Jalal | 57 | 43 | 14 | 26 | 35 ab |
| Azam | 57 | 43 | 14 | 0 | 29 b |
| Salinity Levels means | 67 a | 48 a | 19 b | 18 b | |
Means tailed by different letter (s) are significantly different
In seedling establishment and crop stand of any crop, the germination and emergence of seed is the first important step which indicates attainment of crop production on soils affected by salts. In general, the stress of salts at germination stage delays the start, do not give water to seeds due to osmosis, lessens the rate and increases the dispersion of soil particles which do not allow germination and emergence (Sharif et al., 1999; Cicek and Cakirlar, 2002; Khan et al., 2003; Farsiani and Ghobadi, 2009; Hussain et al., 2010; Abbasi et al., 2012). Hussain et al. (2010) reported that sprouting, rise and growth of young seedlings are highly sensitive to salts stresses than advanced and later progressive stages.
2nd Data on emergence (%) (after completion of emergence): After completion of emergence, the data was collected and converted to percent emergence and revealed that there was significant (p ≤ 0.05) variation among the genotypes’ means. The supreme (96 %) emergence was recorded by Iqbal followed by Jalal genotypes, while minimum by Azam genotypes (81 %) (Table 3). Salinity levels has also affected significantly (p ≤ 0.01) % emergence of maize genotypes. The significantly lowest (84 %) emergence was recorded in salinity of 7.6 dS m-1. The interactions between salinity levels and maize genotype were non-significant.
Table 3: Second Percent emergence (after completion of appearance) of maize genotypes as affected by various induced salinity levels.
| Maize genotypes | Salinity levels (dS m-1) | Means of genotypes | |||
| 0.4 | 2.7 | 5.2 | 7.6 | ||
| Percent (%) emergence | |||||
| Iqbal | 100 | 100 | 98 | 86 | 96 a |
| Jalal | 100 | 100 | 96 | 84 | 95 a |
| Azam | 100 | 71 | 70 | 82 | 81 b |
| Salinity Levels means | 100 a | 90ab | 88ab | 84b | |
Means tailed by unlike letter (s) are significantly different.
The high concentrations of salts create both physiological and morphological stresses, aggravate the rate of sprouting, percentage and disturb the initiation of seedlings from embryo inside the soil by making them weakness (Sharif et al., 1999; Ashraf and Foolad, 2005; Eker et al., 2006; Farsiani and Ghobadi, 2009; Hussain et al., 2010; Akram et al., 2010a; Zahoor et al., 2011; Abbasi et al., 2012).
Number of leaves (No)
The data on number of leaves showed that the variances among the averages of maize genotypes, salinity and interactions between genotypes and salinity levels were non-significantly (Table 4).
Table 4: Number of leaves of maize as influenced by various induced salinity levels.
| Maize Genotypes | Salinity levels (dS m-1) | Means of Genotypes | ||||
| 0.4 | 2.7 | 5.2 | 7.6 | |||
| Number of leaves (No) | ||||||
| Iqbal | 11 | 10 | 9 | 9 | 10 | |
| Jalal | 10 | 9 | 9 | 8 | 9 | |
| Azam | 9 | 9 | 9 | 8 | 9 | |
| Salinity Levels’ means | 10 | 9 | 9 | 8 | ||
Plant height (cm)
The growth (height) of maize genotypes revealed that there was significant (p ≤ 0.05) variation among the means of maize genotypes (Table 5) and means height of maize genotypes are overlapping with each other. The significantly maximum growth in the form of height (94 cm) was documented by Iqbal genotypes followed by Azam (87) and Jalal genotypes (83 cm) (Table 5). The salinity levels have affected considerably (p ≤ 0.01) the growth in the form of height of three maize genotypes. Significantly minimum height (81cm) of maize genotypes was found in salinity level of 7.6 dS m-1 followed by 5.2 dS m-1, while maximum in 0.4 dS m-1 salinity. The interactions were found non-significant. The salinity has affected the morphological attributes of maize, reduced with increasing level of salinity (Eker et al., 2006; Collado et al., 2010; Hussain et al., 2010; Abbasi et al., 2012; Usman et al., 2012). The salinity stresses mainly affect the water absorption, morphological and physiological process i.e. respiration, photosynthesis and metabolism of carbohydrates, protein resulting in decrease of plant growth and development (Sharif et al., 1999; Cicek and Cakirlar, 2002; Khan et al., 2003; Karmaker et al., 2008; Murat at al., 2010; Khodarahmpour, 2011; Abbasi et al., 2012; Khodarahmpour, 2012). Sharif et al. (1999), Davenport et al. (2005) and Quintero et al. (2007) reported that the poison’s levels of salinity and sodicity in different parts of plants damaged the tissues, cells and organelles decreasing the plant progress in the form of height.
Table 5: Plant height (cm) of maize genotypes as influenced by various induced salinity levels.
| Maize genotypes | Salinity levels (dS m-1) | Means of genotypes | |||
| 0.4 | 2.7 | 5.2 | 7.6 | ||
| Plant height (cm) | |||||
| Iqbal | 97 | 97 | 94 | 86 | 94 a |
| Jalal | 93 | 88 | 77 | 73 | 83 ab |
| Azam | 94 | 93 | 77 | 84 | 87 b |
| Salinity Levels means | 95 a | 93 a | 83 b | 83 b | |
Means tailed by unlike letter (s) are significantly different.
Further, salt stress reduces plant growth due to reduction in photosynthetic activities such as the electron transference via contraction of the intracellular space that are related with the outflow of H2O through water passages in the plasma membrane/tissue of crop plants (Greenway and Munns, 1980; Allakhverdier et al., 2000; Silveira et al., 2001; Eker et al., 2006).
Table 6: Shoots weight (g pot-1) of maize genotypes as affected by various induced salinity levels.
| Maize Genotypes | Salinity levels (SL) dS m-1 | Means of Genotypes | |||
| 0.4 | 2.7 | 5.2 | 7.6 | ||
| Shoot weight (g pot-1) | |||||
| Iqbal | 65 | 65 | 64 | 43 | 59 a |
| Jalal | 63 | 55 | 53 | 32 | 51 ab |
| Azam | 52 | 54 | 39 | 36 | 45 b |
| Salinity Levels’ Means | 60 a | 58 a | 52 a | 37 b | |
Means tailed by unlike letter (s) are significantly different.
Shoots weight (g pot-1)
The data on shoots weight (g pot-1) showed that a significant (p ≤ 0.05) variation was detected among the means of maize varieties. The significant maximum shoot weight (59 g/pot) was noted in Iqbal genotypes followed by Jalal (51 g/pot) and minimum (45 g/pot) by Azam variety (Table 6). The salinity levels also affected significantly (p < 0.01) the shoot weight of maize genotypes. Irrespective of maize genotypes, the significantly minimum shoot weight (37 g per pot) was recorded in the pots having salinity of 7.6 dS m-1 followed by 5.2 dS m-1 salinity. The maximum shoot weight (60 g per pot) of maize was noted at 0.4 dS m-1 salinity. The shoot weight produced at 0.4, 2.7 and 5.2 dS m-1 were statically at par. The interaction between the shoot weight of salinity levels and varieties were non-significant (Table 6). Turan et al. (2007) and Ahmed et al. (2012) stated that high concentration of sodium chloride reduced the dry mass as well as the shoots and roots of maize crops severely. The toxicity of salts inhibited the shoots enlargement corn by devastating leaves initiation, elongation, enlargement and internode growth (Sharif et al., 1999; Eker et al., 2006; Akram et al., 2010b; Collado et al., 2010; Hussain et al., 2010; Zahoor et al., 2011; Qu et al., 2012; Usman et al., 2012). Salinity declines the growth rate of leaves and stems in maize (Munns, 1993; Sharif et al., 1999; Cicek and Cakirlar, 2002; Khan et al., 2003; Karmaker et al., 2008; Collado et al., 2010; Murat et al., 2010; Khodarahmpour, 2011; Abbasi et al., 2012; Khodarahmpour, 2012) due to less production of cells and cell elongation (Sharif et al., 1999; Szalai and Janda, 2009). Salt stresses in plants have the capacity to reduce the plant growth through various ways i.e. reduction in photosynthetic activities and the electron transmission by contraction of the intracellular space which is mostly related to the outflow of H2O through waterways in the plasma tissues (Greenway and Munns, 1980; Allakhverdier et al., 2000; Cicek and Cakirlar, 2002).
Table 7: Roots weight (g pot-1) of maize genotypes as affected by various induced salinity levels.
| Maize Genotypes | Salinity levels (dS m-1) | Means of Genotypes | |||
| 0.4 | 2.7 | 5.2 | 7.6 | ||
| Root weight (g pot-1) | |||||
| Iqbal | 13 | 11 | 7 | 5 | 9 |
| Jalal | 9 | 8 | 6 | 4 | 7 |
| Azam | 11 | 9 | 5 | 4 | 7 |
| Salinity Levels’ Means | 11a | 9 bc | 6 bc | 4 c | |
Means tailed by unlike letter (s) are significantly different.
Roots weight (g pot-1)
The data collected on roots weight (g pot-1) indicated that the root weight was influenced significantly (p ≤ 0.05) by altered salinity levels (Table 7). The significantly minimum root weight (4 g/pot) was observed in salinity level of 7.6 dS m-1 followed by salinity level of 5.2 dS m-1 while the supreme root weight (11 g/pot) was noted in 0.4 dS m-1 (Table 7). As regards maize cultivars, there were non-significant differences among the root weight of maize grown under various salinity levels. The interactions of root weight between salinity levels and maize genotypes were non-significant.
According to the reports of Sharif et al. (1999), Khan et al. (2003), Turan et al. (2007), Tas and Basar (2009), Ahmed et al. (2012) and Parvaiz (2014), the shoots and roots biomass of the maize plant are severely declined with the application of high levels of applied NaCl salts. Usman et al. (2012) examined the consequence of sodium chloride salt on morphological attributes of corn and found great variation in the morphological parameters by low production of shoots, roots, length of plant, fresh and dry weight with increased level of salt stress (Cicek and Cakirlar, 2002; Eker et al., 2006; Hussain et al., 2010).
Conclusions and Recommendations
From the results of the study, it is clear that the percent emergence, plant height, shoots and roots weight of maize genotypes were significantly decreased with the linear increasing level of salinity. Further, the results showed that irrespective of salinity levels, the mean maximum emergence, the maximum plant height and shoot weight was documented in Iqbal genotype followed by Jalal genotype. It is concluded from these results that Iqbal genotype morphologically performed better at each salinity level means said to be a tolerant one and can be grown in the saline areas of the central Khyber Pakhtunkhwa (KPK) of Pakistan as well as for general cultivation of maize followed by Jalal genotype. Thus, the cultivation of Iqbal genotype in salt stress conditions of KPK is recommended for maize production.
Acknowledgements
The authors are highly thankful to the administration of Amir Muhammad Khan Campus Mardan, for permission and provision of facilities for the conduction of this piece of research in pots.
Author’s Contribution
Shamsher Ali: Conceived the idea, planned the experiment and overall management of experiment and research article.
M.J. Khan: Helped in writing of material and methods.
Zahir Shah: Helped in data analysis and did proof reading.
Naveedullah: Collected and reviewed the literature.
Abdul Jalal: Conducted the experiment and collected data.
References
Abbasi, G.H., J. Akhtar, M.A.U. Haq and N. Ahmad. 2012. Screening of maize hybrids for salt tolerance at seedling stage under hydroponic condition. Soil Environ. 31(1): 83-90.
Ahmed, K., M. Saqib, J. Akhtar and R. Ahmad. 2012. Evaluation and characterization of genetic variation in maize (Zea mays L.) for salinity tolerance. Pak. J. Agric. Sci. 49(4): 521-526.
Akram, M., M.Y. Ashirf, R. Ahmad, E.A. Warichi, J. Iqbal and M. Mohsen. 2010a. Screening for salt tolerance in maize (Zea mays L.) hybrids at an early seedling stage. Pak. J. Bot. 42(1): 141-154.
Akram, M., M.Y. Ashraf, R. Ahmad, M. Rafiq, I. Ahmad and J. Iqbal. 2010b. Allometry and yield components of maize (Zea mays L.) hybrids to various potassium levels under saline conditions. Arch. Biol. Sci. Belgrade. 62: 1053–1061. https://doi.org/10.2298/ABS1004053A
Allakhverdiev, S.I., A. Sakamoto, Y. Nishiyama, M. Inaba and N. Murata. 2000. Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol. 123: 1047-1056. https://doi.org/10.1104/pp.123.3.1047
Ashraf, M. and M.R. Foolad. 2005. Pre-sowing seed treatment-a short gun approach to improve germination growth and crop yield under saline and none-saline condition. Adv. Agron. 88: 223-271. https://doi.org/10.1016/S0065-2113(05)88006-X
Boyer, J.S. 1982. Plant productivity and environment. Sci. 218: 443-448. https://doi.org/10.1126/science.218.4571.443
Cha-Um, S., Y. Pokasombat and C. Kirdmanee. 2011. Remediation of salt-affected soil by gypsum and farmyard manure importance for the production of Jasmine rice. Aust. J. Crop. Sci. 5: 458-465.
Chinnusamy, V., A. Tazendorf and J.K. Zhu. 2005. Understanding and improving salt tolerance in plant. Crop Sci. 45: 437-448. https://doi.org/10.2135/cropsci2005.0437
Cicek, N. and H. Cakirlar. 2002. The effect of salinity on some physiological parameters in two maize cultivars. J. plant physiol. 28(1–2): 66–74.
Collado, M.B., M.J. Arturi, M.B. Aulicino and M.C. Molina. 2010. Identification of salt tolerance in seedling of maize (Zea mays L.) with the cell membrane stability trait. Inter. Res. J. Plant Sci. 1: 126-132.
Davenport, R., R.A. James, A. Zakrisson-Plogander, M. Tester and R. Munns. 2005. Control of sodium transport in durum wheat. Plant Physiol. 137: 807–818. https://doi.org/10.1104/pp.104.057307
De Azevedo Neto, A.D., J.T. Prisco, J. Eneas, C.E.B. de Abreu and E.G. Filho. 2006. Effect of salt stress on antioxidative enzymes and lipids peroxidation in leaves and roots of salt tolerant and salt sensitive maize varieties. Environ. Exp. Bot. 56: 87-94. https://doi.org/10.1016/j.envexpbot.2005.01.008
Eker, S., G. Comertpay, O. Konufikan, A.C. Ulger, L. Ozturk and S. Cakmak. 2006. Effect of salinity stress on dry matter production and ion accumulation in hybrid maize varieties. Turk. J. Agric. 30: 365-373.
FAO. 2005. Global network on integrated soil management for sustainable use of salt affected soils. FAO Land Plant Nutr. Manage. Ser. Rome, Italy.
Farsiani, A. and M.E. Ghobadi. 2009. Effect of PEG and NaCl stress on two cultivars of corn (Zea mays L.) at germination and early seedling stage. World Acad. Sci. Eng. Technol. 57: 382-385.
Greenway, H. and R. Munns. 1980. Mechanism of salt tolerance in non-halophytes. Annu. Rev. Plant Physiol. 31: 149-190. https://doi.org/10.1146/annurev.pp.31.060180.001053
Hussain, K., A. Majeed, K. Nawaz and M.F. Nisar. 2010. Changes in morphological attributes of maize (Zea mays L.) under NaCl salinity. Am. Eurasian J. Agric. Environ. Sci. 8(2): 230-232.
Karmoker, J.L., S. Farhana and P. Rashid. 2008. Effects of salinity onion accumulation in maize (Zea mays L.) (Cv. bari-7). 37(2): 203-205.
Khan, A.A., A.R. Sajjad and T. McNeilly. 2003. Assessment of salinity tolerance based upon seedling root growth response functions in maize (Zea mays L.). Euphytica. 131: 81-89. https://doi.org/10.1023/A:1023054706489
Khodarahmpour, Z. 2011. Screening maize (Zea mays L.) hybrids for salt stress tolerance at germination stage. Afr. J. Biotechnol. 10 (71): 15959-15965. https://doi.org/10.5897/AJB11.2493
Khodarahmpour, Z. 2012. Evaluation of salinity effects on germination and early growth of maize (Zea mays L.) hybrids. Afr. J. Agric. Res. 7(12): 1926- 1930. https://doi.org/10.5897/AJAR11.1600
Maas, E.V., G.J. Haffman, G.D. Chaba, J.A. Poss and M.C. Shannon. 1983. Salt sensitivity of corn at various growth stages. Irrig. Sci. 4: 45-57. https://doi.org/10.1007/BF00285556
Mansour, M.M.F., K.H.A. Salman, F.Z.M. Ali and A.F.A. Hadid. 2005. Cell and plant response to NaCl in Zea mays cultivars differing in salt tolerance. Gen. Appl. Plant Physiol. 31: 29-41.
Munns, R. 1993. Physiological processes limiting growth in saline soils: some dogmas and hypotheses. Plant Cell Environ. 16: 15–24. https://doi.org/10.1111/j.1365-3040.1993.tb00840.x
Munns, R. 2002. Comparative physiology of salt and water stress. Plant Cell Environ. 25: 239-250. https://doi.org/10.1046/j.0016-8025.2001.00808.x
Munns, R. 2005. Genes and salt tolerance: bringing them together. New Phytol. 167-663. https://doi.org/10.1111/j.1469-8137.2005.01487.x
Murat. A, T. Abdelkarim Eah Nilgun and T. Suleyman. 2010. Effect of salt stress on growth and ion distribution and accumulation in shoot and root of maize plant. Afr. J. Agric. Res. 5(7): 584-588.
Parvaiz, M. 2014. Response of maize to salt stresses a critical review. Int. J. Healthc. Sci. (IJHC) 1 (1): 13-25
Qu, C., C. Lium, X. Gongm, C. Li, M. Hong, L. Wang and F. Hong. 2012. Impairment of maize seedling photosynthesis caused by a combination of potassium deficiency and salt stress. Environ. Exp. Bot. 75: 134–141. https://doi.org/10.1016/j.envexpbot.2011.08.019
Quintero, J.M., J.M. Fournier and M. Benlloch. 2007. Na+ accumulation in shoot is related to water transport in K+-starved sunflower plants but not in plants with a normal K+ status. J. Plant Physiol. 164: 60–67. https://doi.org/10.1016/j.jplph.2005.10.010
Richards, L.A. 1954. Diagnosis and improvement of saline and alkali soils, U.S. Dep. Agric. hand book, no. 60: pp. 136-147.
Ryan, J., G. Estefan and R. Sommer. 2013. Soil and laboratory manual. 2nd Edition. Jointly published by the Internatinal Center for Agricultural Research in the Dry Areas (ICARDA) and the National Agric. Res. Cent. (NARC). pp. 27-75.
Sharif, A.E., Rasul, A. Nisar and M. Sadiq. 1999. Response of maize (Zea mays L.) genotypes to NaCl salinity at various growth stages of crop. Pak. J. Bio. Sci. 23: 606-608.
Silveira. J., M. Arb, V. Ra and O. Jta. 2001. Salinity-induced effects on nitrogen assimilation related to growth in cowpea plants. Environ. Exp. Bot. 46: 171-179. https://doi.org/10.1016/S0098-8472(01)00095-8
Steel, R.G.D. and J.H. Torrie. 1984. Principles and procedures of statistics: A Biometrical Approach. McGraw-Hill, New York, N.Y. 2nd edition. pp. 633.
Szalai, G. and T. Janda. 2009. Effect of salt stress on the salicylic acid synthesis in young maize (Zea mays L.) plants. J. Agron. Crop Sci. 195: 165–171. https://doi.org/10.1111/j.1439-037X.2008.00352.x
Tas, B. and H. Basar. 2009. Effects of various salt compounds and their combinations on growth and stress indicators in maize. Afr. J. Agric. Res. 4 (3): 156-161.
Turan. M., N. Turkmen and N. Taban. 2007. Effect of NaCl on stomata resistance and proline, chlorophyll, Na, Cl and K concentrations of lentil plants. J. Agron. 6: 378-381. https://doi.org/10.3923/ja.2007.378.381
Usman, M., A.U. Haq, T. Ahsan, S. Amjad, Z. Riast and M. Umar. 2012. Effect of NaCl on morphological attributes of maize (Zea mays L.). World J. Agric. Sci. 8(4): 381-384.
Zahoor, M., R. Khaliq, Z.U. Zafar and H.U.R. Athar. 2011. Degree of salt tolerance in some newly developed maize (Zea mays L.) varieties. Iran. J. Plant Physiol. 1(4): 223-232.
To share on other social networks, click on any share button. What are these?








