Evaluation of Common Beans (Phaseolus vulgaris L.) Land Races based on Qualitative Traits Derived from Himalayan, Andean and Mesoamerican regions
Research Article
Evaluation of Common Beans (Phaseolus vulgaris L.) Land Races based on Qualitative Traits Derived from Himalayan, Andean and Mesoamerican regions
Iffat Nawaz1*, Tahseen Zeb1, Bibi Saima Zeb2 and Javaria Sherani3
1Agricultural Research Station Baffa, Mansehra, (KP); Pakistan; 2Department of Environmental Sciences, COMSATS University Islamabad, Abbottabad Campus, Pakistan; 3Department of Horticulture, Faculty of Agricultural Sciences, Ghazi University, Dera Ghazi Khan; Pakistan.
Abstract | Common beans (Phaseolus vulgaris L.) being dual purpose (vegetable plus legume) is grown worldwide and in Pakistan specifically in the Himalayan range. Local landraces cultivated in traditional farming systems are the sole source of its production. 108 land races of beans including 96 landraces of Himalayan region, the ten Mesoamerican and the two Andean genotypes were evaluated at three locations during 2015 and 2016 at the Summer Agricultural Research Station Kaghan, Batakundi Potato Seed Farm Batakundi and Agricultural Research Station Baffa, Mansehra. This two year study is pioneer in Pakistan with an objective to document the characteristics of common bean landraces originating from the three different regions (Himalayan, Mesoamerican and Andean). Furthermore, diversity in qualitative traits (leaf color, leaf pubescence, hypocotyls pigmentation, flower color, pod color, growth habit, dry pod color, pod curvature, pod beak position, pod beak orientation, seed shape, seed coat pattern and seed color) was studied by using standard Chi square test for homogeneity of populations. Results showed that agro climatic conditions have no influence on the qualitative traits. Secondly, each landrace has its own specific and distinguishing trait like flower color, growth habit. Moreover, the traits were found highly heritable and genetically controlled as no environmental influence was observed based on two years data. Therefore, these traits can be considered to identify and distinguish landraces from each other, may be used as morphological markers in maintaining germplasm purity.
Received | June 14, 2021; Accepted | November 05, 2021; Published | December 20, 2021
*Correspondence | Iffat Nawaz, Agricultural Research Station Baffa, Mansehra, Pakistan; Email: iffat_nawaz@yahoo.com
Citation | Nawaz, I., T. Zeb, B.S. Zeb and J. Sherani. 2022. Evaluation of Common Beans (Phaseolus vulgaris L.) Land Races based on Qualitative Traits Derived from Himalayan, Andean and Mesoamerican regions.. Sarhad Journal of Agriculture, 38(1): 275-286.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.1.275.286
Keywords | Common beans, Landraces, Himalayan region, Qualitative traits, Morphological markers
Introduction
Common bean (Phaseolus vulgaris L.) is an annual, predominantly self-pollinated leguminous crop grown worldwide in a broad range of environments and cropping systems (Fetahu et al., 2014). The rate of outcrossing in common bean is below 5% (Gepts et al., 2008). It is morphologically diverse crop with distinguishable qualitative and variable quantitative traits (Joshi et al., 2009). Generally, genetic variation in bean’s landraces is considerably high and has the most diverse population of cultivated crops (Frankel et al., 1995; Qualset et al., 1997). Qualitative traits are deemed useful for assessment of genetic diversity and its relationship in different crop species. High morphological diversity among genotypes is helpful in recombination of genotypes for important economic qualitative and quantitative traits (Balkaya et al., 2005). Therefore, due to high genetic diversity, the landraces are considered as a valuable source of novel genes for the development and maintenance of improved crop cultivars, conserved and protected for future generations (Soleri and Smith, 1995).
Plant landraces represent a repository of a gene pool, local adaptation of domestic species, and thereby are a great source of genetic variations. Such genetic variation can be helpful to mitigate the current and future food challenges. The nature and magnitude of genetic diversity present in any crop species is a key element for the conservation and utilization of genetic resources. Consequently, characterization and differentiation of the native landraces, being a primary source of diversity, is important step in crop improvement and breeding programs.
The traits to distinguish and characterize landrace are seed color, seed shape, growth habit, pod color and flower color. Pubescence of leaf, stem and pod is one of qualitative traits in common beans which protect it from insects/ pests. The common beans varies morphologically in growth type, vegetative traits, pigmentation, flowers, pods and seed characteristics (Singh, 2001a; 1982; Singh et al., 1991b) and these differences are used as tools for crop improvement strategies.
In Pakistan, common bean is a traditional crop. A number of landraces are grown as sole and intercropped with maize. Seed production is a domesticated practice managed by farmers that needs experience, traditional skills and favorable agro-ecological conditions. Moreover, the economic return from the crop is dependent on achieving the high genetic gain in terms of yield as well as ritual, culinary and market desirable traits (tasteful, easy cooking, thin peel) (Cleveland and Soleri, 2007). High profit can be achieved from utilizing physiological and qualitative traits of the indigenous genetic resources by adapting to the local agro climatic conditions with possible tolerance to biotic and abiotic stresses (Vakali et al., 2009) and uniform structure.
Characterization based on qualitative traits was important to describe the diversity and distinctness of common beans landraces collected from the Himalaya region of Pakistan. The objective of this study was to characterize and differentiate common beans landraces based on qualitative traits. The characterization focused on the relationship between qualitative traits and geographical origin.
Materials and Methods
Meteorological data
The Meteorological data of experimental sites (the Summer Agricultural Research Station Kaghan, Batakundi Potato Seed Farm Batakundi and Agricultural Research Station Baffa, Mansehra) is given in Table 1.
Plant materials
Plant material comprised a total of108 accessions, out of which 96 landraces collected from the remote and hilly areas of Pakistan, situated in the Himalaya region and 12 were exotic accessions including the 10 Mesoamerican and the two Andean genotypes imported from Washington State University USA (Table 2). Indigenous experimental material was planted at Summer Agricultural Research Station Kaghan for uniformity and seed multiplication. The exotic accessions represent the Mesoamerican and Andean centers of domestication including Mexico, Bolivia, Columbia and Peru etc.
Experimental Sites, design and procedure
During 2014, the experimental material was collected from the Himalaya region of Pakistan and the single seed of each genotype was planted at the Summer Agricultural Research Station, Kaghan to get uniformity and seed multiplication. 108 including 96 landraces of Himalayan region, the ten Mesoamerican and the two Andean genotypes were evaluated at three locations during 2015 and 2016. The experiments were planted during the cropping season of 2015 and 2016 at the Summer Agricultural Research Station Kaghan, Batakundi Potato Seed Farm Batakundi and Agricultural Research Station Baffa, Mansehra. Field trials were planted in the Alpha Lattice design with three replications. The crop was sown in the month of June in both years in 2 rows of 5m length with 60cm row spacing and 35cm plant to plant distance. All the standard crop husbandry practices were applied throughout the cropping season. Harvesting was done at maturity of accessions.
Traits measurements
Data were recorded for 13 qualitative traits i.e. Leaf color, hypocotyls pigmentation, leaf pubescence, growth habit, flower color, pod color, dry pod color, pod curvature, pod beak position, pod beak orientation, seed coat pattern, seed color and seed shape. Out of which, three traits were binary while 10 traits were nominal. Data were recorded according to the
Table 1: Meteorological Data of Experimental Sites.
|
Location |
Geographic position |
Months |
Temperature oC |
||||
|
Kaghan |
Longitude |
Latitude |
Elevation |
2015 |
Min |
Max |
Ave |
|
73.5253o |
34.7768 o |
2108.83m |
June |
18 |
31 |
25 |
|
|
July |
20 |
30 |
25 |
||||
|
August |
19 |
30 |
24 |
||||
|
September |
16 |
28 |
21 |
||||
|
October |
12 |
24 |
17 |
||||
|
2016 |
|||||||
|
June |
20 |
34 |
27 |
||||
|
July |
20 |
31 |
25 |
||||
|
Augest |
19 |
30 |
24 |
||||
|
September |
17 |
30 |
23 |
||||
|
October |
13 |
20 |
18 |
||||
|
Batta Kundi |
2015 |
||||||
|
73.774262 o |
34.931567 o |
2659m |
June |
18 |
31 |
25 |
|
|
July |
20 |
30 |
25 |
||||
|
August |
19 |
30 |
24 |
||||
|
September |
16 |
28 |
21 |
||||
|
October |
12 |
24 |
17 |
||||
|
2016 |
|||||||
|
June |
20 |
34 |
27 |
||||
|
July |
20 |
31 |
25 |
||||
|
August |
19 |
30 |
24 |
||||
|
September |
17 |
30 |
23 |
||||
|
October |
13 |
26 |
18 |
||||
|
Baffa |
2015 |
||||||
|
73.2194 o |
34.4378 o |
920.49m |
June |
25 |
37 |
31 |
|
|
July |
27 |
36 |
37 |
||||
|
August |
25 |
35 |
30 |
||||
|
September |
21 |
33 |
27 |
||||
|
October |
18 |
29 |
22 |
||||
|
November |
13 |
23 |
16 |
||||
|
2016 |
|||||||
|
June |
27 |
39 |
33 |
||||
|
July |
27 |
37 |
32 |
||||
|
August |
25 |
36 |
30 |
||||
|
September |
24 |
35 |
29 |
||||
|
October |
19 |
32 |
24 |
||||
|
November |
14 |
26 |
18 |
||||
Source: Meteorological department Khyber Pakthunkhwa Pakistan
International Board of Plant Genetic Resources (IBPGR, 1982) descriptors of Phaseolus vulgaris with some modifications in a single replicate according to Singh et al. (1991a; 1991b).
Leaf color was observed during the plant growth period at two-leaf stage. It was scored on scale light to dark to light according to the IBPGR (1981). One observation per plot was taken in a single replicate. Hypocotyls pigmentation of stem was recorded one week after 50% sprouting. It was scored as (1) for presence of anthocyanin pigmentation in
Table 2: List of Plant material.
|
G1: Landraces from the Himalaya Region of Pakistan. |
|||
|
Genotype code |
Genotype code |
Genotype code |
Genotype code |
|
SnMLB1 |
KNLCBBS25 |
StR49 |
ShLSMS73 |
|
SnKLB2 |
GtGBBS26 |
KtR50 |
KrSRS74 |
|
KnCkLB3 |
ChPBBS27 |
MnR51 |
NrPSP75 |
|
BALB4 |
KrSBS28 |
PcR52 |
BKbPSP76 |
|
MALB5 |
BASBS29 |
MnBR53 |
KrPSS77 |
|
GtGB6 |
KtSBS30 |
KtR54 |
BkPSP78 |
|
ChMLB7 |
UdSY31 |
PcDM55 |
StPBS79 |
|
ChPS8 |
NrR32 |
KtM56 |
KtPBS80 |
|
ChBLB9 |
MDR33 |
KtRB57 |
ChMTP81 |
|
ShLS10 |
GtGR34 |
KtPB58 |
ChBLP82 |
|
KrB11 |
ChMR35 |
SnMB59 |
PcW83 |
|
BAS12 |
ChPR36 |
GtGB60 |
ShLW84 |
|
KtLB13 |
ChBR37 |
ChMB61 |
KrW85 |
|
StLB14 |
ShLR38 |
ChBSB62 |
BAW86 |
|
BADB15 |
KrR39 |
ShLBsh63 |
KtW87 |
|
PcB16 |
BAR40 |
KrBsh64 |
PcWBD88 |
|
KtS17 |
UdSR41 |
BAB65 |
AJKBBD89 |
|
SnMBBS18 |
MnR42 |
PcB66 |
NARCBBD90 |
|
KnCkBBS19 |
MnR43 |
StB67 |
GtGGB91 |
|
KnIcBBS20 |
MnR44 |
KtB68 |
GtGGB92 |
|
ShLBBS21 |
StR45 |
SnKSBS69 |
GtGBGS93 |
|
KrBBS22 |
StR46 |
SnKLBBS70 |
GtGBPS94 |
|
BABBS23 |
StR47 |
KnChLBRS71 |
GtGPB95 |
|
KtBBS24 |
StR48 |
GtGLBMS72 |
KrSBS96 |
|
G2: The Mesoamerican genotypes |
|||
|
MHBrM103 |
MMSBrM113 |
MDSBrM124 |
ESDMM128 |
|
MABrM112 |
CRBM121 |
GtWM123 |
MGBrdBrStM136 |
|
MPBM140 |
MSYSM143 |
||
|
G3: The Andean Genotypes |
|||
|
PuBA129 |
PLYA142 |
||
hypocotyls and (0) for absence of pigment by visual observation through naked eye. Leaf pubescence was observed through the magnifying glass. It was scored (1) for presence of pubescence while (0) for absence of pubescence at leaf surface. Growth habit was recorded according to two major types i.e. bush and climbing type. Flower color of each entry was observed at peak flowering stage in freshly opened flowers under the natural daylight conditions. The flower color was classified as purple and white. The color observed when immature pods were fully expanded under natural day light condition. Pod color was classified as light green, green, green with purple shade, green with purple stripes, green with red stripes and light green with purple shade. Pod curvature was noted according to the IBPGR descriptor of fully expanded immature pods. It was observed according to three categories i.e. curved, semi-curved and straight. Pod beak orientation was observed of fully expanded immature pods and classified as straight, upward and downward.
Seed color was noted after threshing as it became constant after threshing. 39 colors were observed after threshing the dried pods of 108 different common beans accessions belonging to three groups i.e. the landraces of Himalayan Region, the Mesoamerican and the Andean. Pod beak position was noted of fully expanded immature pods according to two categories mentioned in the IBPGR descriptor i.e. marginal and nonmarginal. The seed coat pattern was identified of seed after harvest following IBPGR descriptors. The color of dry pods per entry was observed at maturity. Seed shape was observed by using 10X magnifying glasses for seeds taken from the middle of the pod as per IBPGR descriptor. It was recorded at harvesting stage and classified in to four categories i.e. cuboid, oval, kidney and truncate fastigiate.
Statistical analysis
Data recorded on various qualitative traits were statistically analyzed by applying a Chi- square test (Pearson, 1900) for homogeneity of the population using the Minitab Statistical Software. The chi- square test is based on observed values (Oi) and expected values (Ei) for traits and can be represented as;
Chi-square statistic:
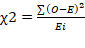
Results and Discussion
Homogeneity test (Chi-square)
Homogeneity test (Chi-square) values are shown in Table 3 for all 13 qualitative traits. Chi- square calculated value was higher than tabulated for only leaf color. It showed that leaf color among three groups was significant showing that leaf color may have some relationship with source of collection. Considering the other 12 traits including hypocotyls pigmentation, leaf pubescence, growth habit, flower color, pod color, dry pod color, pod curvature, pod beak position, pod beak orientation, seed coat pattern, seed color and
Table 3: Homogeneity test (chi square) values for the examined qualitative traits.
|
S.No |
Qualitative trait |
Df |
χ2 calculated |
χ2 tabulated |
|
1 |
Leaf color |
6 |
18.37 |
16.81 |
|
2 |
Hypocotyls pigmentation |
2 |
2.56 |
9.21 |
|
3 |
Leaf pubescence |
2 |
0.79 |
9.21 |
|
4 |
Growth habit |
2 |
0.38 |
9.21 |
|
5 |
Flower color |
2 |
2.86 |
9.21 |
|
6 |
Pod color |
10 |
5.13 |
23.21 |
|
7 |
Pod curvature |
4 |
3.53 |
13.28 |
|
8 |
Pod beak orientation |
4 |
2.35 |
13.28 |
|
9 |
Pod beak position |
2 |
3.41 |
9.21 |
|
10 |
Dry pod color |
10 |
7.63 |
23.21 |
|
11 |
Seed coat pattern |
10 |
4.79 |
23.21 |
|
12 |
Seed color |
76 |
101.91 |
107.58 |
|
13 |
Seed shape |
6 |
12.16 |
16.81 |
seed shape, Table 3 depicted that chi-square calculated values for all traits were less than the chi-square tabulated values describing non-significant results. These results revealed that there was no relationship among 12 qualitative traits of common bean landraces and its source of collection.
Frequency distribution of qualitative traits
All qualitative traits showed a wide variation among 108 accessions evaluated during this study. These 108 common beans accessions were divided into three groups based on their site/ source of collection i.e. G1 consisted of 96 landraces collected from the Himalaya region of Pakistan, G2 contained 10 genotypes from the Mesoamerican gene pool and G3 had only 2 genotypes of the Andean gene pool.
The frequency distribution of the qualitative traits leaf color, hypocotyls pigmentation, leaf pubescence and growth habit is shown in Figure 1. Leaf color was dark green for 43 landraces followed by the medium green (32) and pale green (21) in the landraces group (G1) while the seven Mesoamerican genotypes (G2) had the medium green leaf color, two had dark green and one was having light green leaf color.
In the case of G3 (Andean gene pool), one genotype had the medium green while others had pale green leaf color. Hypocotyls pigmentation was present in the 50 landraces and absent in 46 in G1. Regarding G2, it was absent in six genotypes and present in four genotypes, however, pigmentation was absent in both Andean genotypes of G3. Leaf pubescence was dominant in all these accessions with 90 landraces in G1, 10 genotypes in G2 and 2 genotypes in G3. Leaf surface was glabrous in only six landraces of G1 while no landrace was with glabrous leaf surface in G2 and G3. Growth habit is an important trait in common beans’ breeding programs because it plays a vital role in adaptability according to the need of farmers. Regarding growth habit, the 93 landraces had climbing type growth habit in G1 while only three landraces were bushy. In G2 and G3; all genotypes were having climbing growth habit. White flower color was found in the 50 landraces of G1, seven genotypes of G2 and two genotypes of G3 while purple flower color was observed in the 46 landraces and three genotypes of G1 and G2, respectively (Figure 2). Green pod color was predominant with different intensities like the 49 landraces in G1, 8 genotypes in G2 and one genotype in G3 had green color, while green pod color with purple shade was found in the 17 landraces of G1 and one genotype each from G2 and G3, respectively (Figure 3). 20 landraces of G1 and one genotype of G2 had light green pod color with purple shade. Light green pod color, green pod color with red strips and green pod color with purple strips was observed in five, three and two landraces of G1, respectively.
Out of 96 landraces in G1, the 47 landraces had semi curved, the 33 had straight and the 16 had curved pod curvature (Figure 2). In G2, five genotypes were having semi-curved, three had curved and two had straight pod curvature. Both genotypes of G3 were having semi-curved pod curvature. Marginal pod beak position was dominant with its presence in 91
landraces, eight and two genotypes in G1, G2 and G3 respectively while, nonmarginal pod beak position was found in five landraces of G1 and only two genotypes of G2 (Figure 2). Sixty landraces in G1, eight genotypes in G2 and two genotypes of G3 had downward pod beak orientation while straight pod beak orientation was observed in 35 landraces of G1 and two genotypes of G2. Only one landrace of G1 had upward pod beak orientation. Six dry pod colors were observed with the frequency of the 57 golden, the 27 having the golden color with purple strips, the 9 had the golden color with pinkish shade, two had golden pod color with purple strip and one had golden pod color with red strips in G1 (Figure 3). All the ten genotypes of G2 and both genotypes of G3 had golden dry pod color. The seed coat pattern (Figure 3) was absent in the 50 landraces of G1, five genotypes of G2 and one genotype of G3. Constant mottled seed coat pattern was observed in the 17 landraces of G1, three genotypes of G2 and one genotype of G3. A stripped seed coat pattern was found in the 15 landraces and one genotype of G1 and G2, respectively. In G1, the 9 landraces had rhomboid spotted and two had circular mottling. Speckled seed coat pattern was found in three landraces and one genotype of G1 and G2, respectively. Four different seed shapes (Figure 3) were found with frequency distribution of the 45, the 32, the 14 and the 5 for cuboid, oval, truncate fastigiate and kidney-shaped, respectively in G1. In G2, the 4 genotypes had cuboid seed shape, the 3 were kidney shaped, the 2 genotypes had truncate fastigiated and only 1 genotype had oval seed shape. One genotype had oval seed shape while others had truncate fastigiated seed shape in G3.
A wide range of the seed colors such as red, white, black, brown, skin, yellow, tea pink, light pink, brown with black strips, skin with black strips, maroon along with varying tonalities was obtained, however, frequencies of all colors were shown in Figure 4.
Qualitative traits are the most important heritable characters that are commonly used to differentiate landraces. These include the growth habit, the flower color, the pod color, the seed color and the seed shape. The simplified phenotypic approach using qualitative traits for characterization and discrimination of accessions has been found useful to unravel the level of genetic diversity (Atilla et al., 2010; Szilagyi et al., 2011). The present study investigated variability among the qualitative traits found in the native beans from the Himalaya region of Pakistan and frequency distribution in these traits and relationships among different traits and source of collection.
A wide range of variability was found for qualitative traits among and within groups collected from different agro-ecological regions. The nonsignificant chi-square values showed that there was no relationship between all the traits and different groups of common beans (the landraces (G1), the Mesoamerican (G2) and the Andean (G3) collected from different regions. These results revealed that qualitative traits were widely distributed among accessions irrespective of the origin/place of collection. Accessions belonging to the specific group had differences in the qualitative traits. Boros et al. (2014) also reported that qualitative traits were not linked with the origin. These were distributed among the accessions independent of the distribution of accessions to groups based on the origin of accessions.
The frequency distribution obtained for 13 qualitative traits revealed the maximum possible range of variability for all the accessions. The leaf color showed marked variation ranging from dark green to light green and pale green color. Kiwuka et al. (2012) reported a similar leaf color range in the assessment of common beans cultivar diversity of central Uganda. The predominant leaf color was dark green followed by the medium green, the pale green and the light green. Loko et al. (2018) reported the green, the medium green and the dark green leaf colors, respectively, in the characterization of the common bean landraces. The hypocotyls pigmentation (anthocyanin) was present in almost half of accessions in all groups. Loko et al. (2018) observed the hypocotyls pigmentation (anthocyanin) in all studied landraces of the common beans. The leaf pubescence is an important trait and has a pronounced role in both diseases and insects resistance. It is evident that the leaf pubescence interrupts the fungal spores’ production and can physically wound the insects resulting in decreased predation (Mmbaga and Steadman, 1992). The leaf pubescence was predominant in our study and only six landraces were found glabrous. Plant growth habit is one of most commonly selected traits in common bean improvement programs. Plant growth habit varied from climbing to bushy type. Commonly, the predominance of plant growth habit is related to the cropping system and ecological adaptation. Climbing types are preferred in hilly areas where common beans are intercropped with maize, while bush type is more desirable when common beans are grown as sole crop (Rana et al., 2015). In the present study, both types of plant growth habits were found in indigenous landraces of Himalaya region but frequency of climbing type was high in indigenous landraces and was found a dominant trait. Piergiovanni and Lioli (2010) reported that 90% of Italian landraces found in Basilicata region were climbing type beans. Similarly, Loko et al. (2018) found that all common bean landraces in their study were determinate climbing types. Contrarily to our results, Boros et al. (2014) reported that all accessions of Polish gene banks tested in their experiment had bush growth type. It was also observed in our study that bush-type beans had early maturity, short plant height and low productivity whereas climbing type common beans had longer life cycles, late maturity and high productivity. Similar results were reported by Gracia et al. (1997) and Rana et al. (2015), who observed that climbing types were late maturing and more productive than bushy type. Contradictory findings were due to different genetic makeup of landraces as environment may have negligible effects on qualitative traits.
Plant breeders used flower color along with other distinct qualitative traits as a criterion for varietal purity (Leaky, 1988). Two different flower colors were observed in this study i.e. white and purple. Results showed that white flower color was abundant as compared to the purple flower color. Similarly, Fisseha et al. (2018) also observed white and purple flower color in common beans landraces of Ethiopia. Contrarily to our results, Chhetri and Bhatta (2017) reported a wider range of flower colors in their study while Rana et al. (2015) observed white, pink and lilac flower colors among 4274 accessions with predominance of white color. Pod color ranged from light green to green as well as purple shade on green, purple strips on green and red strips on green color in the current investigation. The frequency of green pod color was high in indigenous landraces as well as in Mesoamerican group. A similar variation in pod color was also reported by Chhetri and Bhatta (2017). Rana et al. (2015) also described that green pod color was predominant with various intensities of dull to shiny green in majority of accessions studied by them. The most abundant pod curvature observed in this study was semi curved followed by straight and curved. Slightly curved pod curvature was also predominant in common beans accessions of Benin Republic (Loko et al., 2018). Similarly, downward pod beak orientation was dominant following straight; however; only one indigenous landrace had upward pod beak orientation. The frequency of marginal pod beak position was very high as compared to non-marginal pod beak position found in only five indigenous landraces and the two Mesoamerican genotypes. Contrarily to this, substantial presence of non-marginal pod beak position was reported in common beans accessions by Loko et al. (2018). Contradictory findings in this regard may be due to differences in genetic makeup of genotypes tested. Dominant dry pod color was golden and found in majority of accessions, whereas golden with purple strips, red strips and golden with purple and pinkish shades were also observed. Loko et al. (2018) observed yellow pigmentation in the pods at physiological maturity stage.
Seed traits are the most important in the common beans especially for commercial acceptability of different varieties Bisht et al. (2014), Rana et al. (2014), (2015). The seed traits have an important role in breeding programs because these traits have been considered as highly heritable Singh et al. (2007), Blair et al. (2010). Marked variations were observed in our study regarding seed color ranging from single color i.e. red, maroon, white, black, brown, skin, to mottled and striped types with different tonalities. Similar observations were reported by Boros et al. (2014), Rana et al. (2015) and Loko et al. (2018). The preference of seed color varied from area to area and region to region throughout the world. In Pakistan, usually red, maroon and mottled and striped seeds with the various tonalities are preferred. Similarly, red, maroon, pink and yellow beans are preferred in India (Rana et al., 2015). The seed color preferences in different parts of the world were also reported by Loko et al. (2018) who stated that brown-red color was dominated in common bean collection from the Benin Republic. Ash colored beans are most preferred, highly priced and widely cultivated in Nilgiris (Jose et al., 2009). Similarly, in Poland white seed color of the Polish local populations of the common beans was highly preferred but some colored beans are also used for various dishes (Boros et al., 2014). The seed characteristics like color, size and shape of common beans are of special attention for consumers and these reflect consumers as well as farmers’ preferences (Stoilova et al., 2013; Loko et al., 2018). Among tested landraces, the cuboid and oval seed shapes were present in high frequency followed by truncate fastigiated and kidney shaped. Contrarily to our observations, Boros et al. (2014) reported high proportion of kidney and round shape than cuboid and oval seed shape in their study. Seed coat pattern was absent in half of landraces as well as Mesoamerican and Andean genotypes. The seed coat patterns observed in this study were constant mottled, stripped, rhomboid spotted, speckled and circular mottling. Boros et al. (2014) also revealed absence of seed coat pattern in majority of local populations of common beans from Polish gene bank with low presence of mottled and stripped seed coat pattern. Similarly, Rana et al. (2015) reported seed coat pattern absence in most of accessions with some extent of mottled seed coat pattern of various colors.
Conclusions and Recommendations
It is evident that considerable diversity was found in the qualitative traits of common bean landraces collected from the Himalaya region of Pakistan. The results of this study revealed major role of the qualitative traits especially growth habit, flower color, leaf pubescence, seed color, seed shape, seed coat pattern and pod related traits in differentiation and characterization of those indigenous landraces. The qualitative traits were found highly distinguishable and heritable being less influenced by the environment as no change was depicted in the studied traits during two years’ experiments at three different locations. These traits may be considered worthwhile in differentiating landraces as well as preferably used by the breeders for maintaining purity of these landraces. Consequently, the landraces with highly desirable traits of agronomic interest can be utilized in breeding programs for crop improvement. As a further step, development of trait specific subsets and core sets are recommended to elaborate more valuable information on overall genetic diversity and particular genes responsible for specific traits of economic interest in present collection.
Acknowledgements
The authors acknowledge the support of Hazara Agricultural Research Station Abbottabad for providing field facilities for experiments at its sub stations of Kaghan and Battakundi.
Novelty Statement
Common beans (Phaseolus vulgaris L.) being the most important source of protein among legumes, is still an unexploited area of study especially in case of indigenous germplasm. Extensive characterization of unexplored landraces is needed to unravel its breeding potential. The results of this study explored that qualitative traits of common beans are highly heritable and can be utilized in the identification of different landraces and their purity maintenance. These qualitative traits can be used as morphological markers in further breeding programs.
Author’s Contribution
Iffat Nawaz: Designed the experiment and performed core research work in this article.
Tahseen Zeb and Bibi Saima Zeb: Helped in manuscript preparation and data interpretation.
Javaria Sherani: Helped in proofreading.
Conflict of interest
The authors have declared no conflict of interest.
References
Atilla, D., K. Haliloglu and M. Ekinci. 2010. Characterization of breeding lines of the common bean as revealed by RAPD and relationship with morphological traits. Pak. J. Bot. 42(6): 3839-3845.
Balkaya, A., R. Yanmaz and A.A. Kar. 2005. Morphological characterization of white head Cabbage (Brassica oleracea var. capitatasubvar. Alba) genotypes in Turkey. N.Z.J. Crop. Hort. Sci., 33: 333-341. https://doi.org/10.1080/01140671.2005.9514367
Bisht, I.S., S.R. Pandravada, J.C. Rana, S.K. Malik, A. Singh, P.B. Singh, F. Ahmad and K.C. Bansal. 2014. Subsistence farming, agrobiodiversity and sustainable agriculture: A case study. J. Agron. Econ. Sustain. Food. Syst., 38(8): 890-912. https://doi.org/10.1080/21683565.2014.901273
Blair, M.W., L.F. Gonzalez, P.M. Kimani, and L. Butare. 2010. Genetic diversity, intergene pool introgression and nutritional quality of common beans (Phaseolus vulgaris L.) from Central Africa. Theor. Appl. Genet., 121: 237- 248. https://doi.org/10.1007/s00122-010-1305-x
Boros, L., A. Wawer and K. Borucka. 2014. Morphological, phenological and agronomical characterization of variability among common bean (Phaseolus vulgaris L.) local populations from the National center for plant genetic resources: Polish genebank. J. Hort. Res. 22(2): 123-130. https://doi.org/10.2478/johr-2014-0029
Chhetri, A. and A. Bhatta. 2017. Agro-morphological variability assessment of common bean (Phaseolus vulgaris L.) genotypes in high hill Jumla, Nepal. Int. J. Environ. Agric. Biotech., 2(6): 3110- 3115. https://doi.org/10.22161/ijeab/2.6.42
Cleveland, D. and D. Soleri. 2007. Farmer knowledge and scientist knowledge in sustainable Agricultural development: ontology, epistemology and praxis. In: P. Sillitoe (ed.). Local Science vs. Global Sci: Approaches to indigenous knowledge in international development. Berghahn Books, New York. pp. 209-229.
Fetahu, S., S. Aliu, I. Rusinovci, A. Behluli and B. Kelmendi. 2014. Genetic diversity for micronutrients contents in some common bean landraces (Phaseolus vulgaris L.). In: Maric. S, Z. Loncaric. Proceedings of 49th Croatian and 9th International Symposium on Agriculture. Dubrovnik, Croatia.
Fisseha, Z., M. Kyallo, K. Tesfaye, J. Harvey, K. Dagne, S. Opyio and P. Gepts. 2018. Integrating phenotypic evaluations with a molecular diversity assessment of an Ethiopian collection of common bean landraces. Afr. Crop. Sci. J., 26(2): 313-324. https://doi.org/10.4314/acsj.v26i2.12
Frankel, O.H., A.H.D. Brown and J.J. Burdon. 1995. The conservation of plant biodiversity.Cambridge Univ. Press. Cambridge.
Gepts, P., F.J.L. Aragao, E. Barros, M.W. Blair, R. Brondani, W. Broughton, I. Galasso, G. Hemandez, J. Kami, P. Lariguet, P. McClean, M. Melotto, P.N. Miklas, P. Pauls, A. Pedrosa-Harand, T. Porch, F. Sanchez, F. Sparvoli and K. Yu. 2008. Genomics of Phaseolus beans, a major source of dietary protein and micronutrients in the Tropics. In: P. H. Moore and R. Ming (Eds). Genom. Trop. Crop. Plants. Spring., pp.113-142. https://doi.org/10.1007/978-0-387-71219-2_5
Gracia, E.H., C.B. Pena-Valdivia, J.R. Rogelio-Aguirre and J.S.M. Muruaga. 1997. Morphological and agronomic traits of a wild population and an improved cultivar of common bean (Phaseolus vulgaris L.). Ann. Bot., 79: 207-213. https://doi.org/10.1006/anbo.1996.0329
IBPGR. 1982. Descriptors for Phaseolus vulgaris L. International Board for Plant Genetic Resources (IBPGR). Rome. Italy.
Jose, F.C., M.M.S. Mohammad, G. Thomas, G. Varghese, N. Selvaraj and M. Dorai. 2009. Genetic diversity and conservation of common bean (Phaseolus vulgaris L.) landraces Nilgiris. Curr. Sci., 97(2): 227-235.
Kiwuka, C.R., Bukenya-Ziraba, M. Namaganda and J.W. Mulumba. 2012. Assessment of Common bean cultivar diversity in selected communities of central Uganda. Afr. Crop. Sci. J., 20(4): 239-249.
Leaky, C.L.A. 1988. Genotypic and phenotypic markers in common bean. In: P. Gepts (ed.). Genetic resources of Phaseolus beans. Kluwer, Dordrecht, Netherlands. pp. 245-327. https://doi.org/10.1007/978-94-009-2786-5_12
Loko, L.E.Y., A. Orobiyi, A. Adjatin, J. Akpo, J. Toffa, G. Djedatin and A. Dansi. 2018. Morphological characterization of common bean (Phaseolus vulgaris L.) landraces of central region of Benin Republic. J. Plant. Breed. Crop. Sci., 10(11): 304-318. https://doi.org/10.5897/JPBCS2018.0766
Mmbaga, T. and J.R. Steadman. 1992. Nonspecific Resistance to Rust in Pubescent and Glabrous common bean genotypes. Phytopath. 82: 1283-1287. https://doi.org/10.1094/Phyto-82-1283
Moody, B.D., L.C. Emebiri, M. Spackman and G.T. Mekuria. 2014. Breeding for qualitative and quantitative traits using markers. Deptt. Prim. Indust. Res. Develop. Div. Horsham, VIC, 3041, Australia.
1900. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 50(302): 157-175. https://doi.org/10.1080/14786440009463897
Piergiovanni, A.R. and L. Lioi. 2010. Italian Common Bean Landraces: History, Genetic Diversity and Seed Quality. Diversity, 2: 837-862. https://doi.org/10.3390/d2060837
Qualset, C.O., A.B. Damania, A.C.A. Zanatta and S.B. Brush. 1997. Locally based crop plant conservation. In N. Maxted et al (ed.). Plant. Genet. Conser. The in situ approach. Chapman and Hall, London. pp. 160-175. https://doi.org/10.1007/978-94-009-1437-7_10
Rana, P.K., P. Kumar, V.K. Singhal and J.C. Rana. 2014. Uses of local plant diversity among the tribal communities of Pangi valley of district Chamba in cold dessert Himalaya, India. World. Sci. J., 753289. https://doi.org/10.1155/2014/753289
Rana, J.C., T.R. Sharma, R.K. Tyagi, R.K. Chahota, N.K. Gautam, M. Singh, P.N. Sharma and S.N. Ojha. 2015. Characterization of 4274 accessions of common bean (Phaseolus vulgaris L.) germplasm conserved in the Indian gene bank for phenological, morphological and agricultural traits. Euphytica., 205: 441-457. https://doi.org/10.1007/s10681-015-1406-3
Singh, S.P. 1982. A key for identification of different growth habits of Phaseolus vulgaris L. Bean. Improv. Coop., 25: 92-94.
Singh, S.P., J.A. Gutierrze, A. Molina, C. Urrea and P. Gepts. 1991b. Genetic diversity in cultivated common bean: II. Marker based analysis of morphological and agronomic traits. Crop Sci., 31: 23-29. https://doi.org/10.2135/cropsci1991.0011183X003100010005x
Singh, S.P. 2001a. Broadening the genetic base of common bean cultivars: a review.Crop. Sci., 41(6): 1659-1675. https://doi.org/10.2135/cropsci2001.1659
Singh, D.P., V.S. Tomar, R.K. Behl, S.D. Upadhyaya, M.S. Bhale and D. Khare. 2007. Crop Production in stress environments: Genetic and management options. Jodpr. Agrbio. Int., 30: 1-656.
Soleri, D. and S.E. Smith. 1995. Morphological and phenological comparisons of two hopi maize Varieties conserved in-situ and ex-situ. Econ. Bot., 49: 56-77. https://doi.org/10.1007/BF02862278
Stoilova, T., G. Periera and M.T. De-Sousa. 2013. Morphological characterization of a small common bean (Phaseolus vulgaris L.) collection under different environments. J. Cent. Europ. Agric.14(3): 854-864. https://doi.org/10.5513/JCEA01/14.3.1277
Szilagyi, I., S. Taygar, M. Ciuca. 2011. Evaluation of genetic diversity in common bean (P. vulgaris L.) using RAPD markers and morpho agronomic traits. Rom. Biotechnol. Lett. 16(1): 98-105.
Vakali, C., F. Papathanasiou, I. Papadopoulos and E. Tamoutsidis. 2009. Preliminary results on a Comparative study evaluating landraces of common bean (Phaseolus vulgaris L.) under organic agriculture in a protected area in Greece. 24th Scientific conference within the framework of the 9th European Summer Academy on organic farming. Lednicena Morave, Czech Republic.
To share on other social networks, click on any share button. What are these?







