Antiviral Potential of Plant Activators and Nutrients for the Management of Potato Leaf Roll Virus
Research Article
Antiviral Potential of Plant Activators and Nutrients for the Management of Potato Leaf Roll Virus
Muhammad Musa1*, Saba Saeed2, Azher Mustafa2, Muhammad Ussama Yasin2, Saima Naseer2 and Iftikhar Haider3
1Ayub Agricultural Research Institute, Faisalabad, Pakistan; 2Virology Section, Plant Pathology Research Institute, AARI, Faisalabad, Pakistan; 3AAE, Ayub Agricultural Research Institute, Faisalabad, Pakistan.
Abstract | Foliar application of five plant defense activators i.e., salicylic acid, citric acid, benzoic acid, KH2PO4 and K2HPO4 were tested against potato leaf roll virus (PLRV) in field conditions while two nutrient solutions i.e., macronutrients (N, P, K) and micronutrients (B, Zn, Cu, Mn, and Fe) were tested against the disease under glass house conditions during the years 2018 and 2019. All plant defense activators and nutrients were sprayed thrice using three different concentrations (0.25%, 0.50%, 0.75%) with 15-days interval. Although all activators were found effective to manage PLRV with some variations, but maximum disease was managed by salicylic acid with 0.75% concentration. In case of nutrients application, both solutions were found to be effective against PLRV but macronutrients solution with 0.75% concentration was found more effective than micronutrients solution to manage PLRV. It is advised to get better results against PLRV that both macro- and micro-nutrients should be sprayed in combination.
Received | July 01, 2021; Accepted | March 20, 2022; Published | March 30, 2022
*Correspondence | Muhammad Musa, Ayub Agricultural Research Institute, Faisalabad, Pakistan; Email: mum96@hotmail.com
Citation | Musa, M., S. Saeed, A. Mustafa, M.U. Yasin, S. Naseer and I. Haider. 2022. Antiviral potential of plant activators and nutrients for the management of potato leaf roll virus. Pakistan Journal of Agricultural Research, 35(1): 209-214.
DOI | https://dx.doi.org/10.17582/journal.pjar/2022/35.1.209.214
Keywords | Concentration, Disease management, Nutrients, Plant defense activators, Potato leaf roll virus
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Potato is the 4th important crop of the world with annual production of 388.2 million tons, grown at an area of 19.3 million hectares (Khalid et al., 2020). The area under potato cultivation in Pakistan was 195.7 thousand hectares with an average production of 24.89 t ha-1 (FAOSTAT, 2020). The attack of various bacterial, fungal and viral diseases is main obstacle in obtaining high yield of potato. Viral diseases are challenging to control for which more attention is needed (Gebhardt and Valkonen, 2001). Among various viral diseases, potato leaf roll is more infectious. In Pakistan, occurrence of PLRV (Potato Leaf Roll Virus) was first reported in 1978 (Ali et al., 2002). In Pakistan, PLRV is reported to be an aggressive disease due to excessive occurrence of 15-65% documented in the area. It is detected through enzyme-linked immunosorbent assay tests (Mughal, 2003). In Pakistan, 70-90% reduction in potato yield has been reported due to PLRV (Bhutta and Bhatti, 2002; Abbas et al., 2012; 2016). It largely affects the tuber and foliage. The virus sticks in phloem tissues, which makes it difficult to reclaim. It also causes necrosis and atypical functioning of carbohydrate that finally disrupt the transport of starch from leaves to tubers. Infected tubers are prime source of infection which result in yield reduction with undersized tubers. It ultimately lessens market value as compared to healthy ones (Anonymous, 2018). Rapid spread of leaf roll occurs through vector of aphid “Myzuspersicae” in spring crop of potato (Saljoqi, 2009).
Many practices are used for the management of PLRV including cultural control, use of chemical, plant extracts as botanical control, use of virus free tubers and resistant cultivars. Insecticides are used to manage the insect vector (Ali et al., 2011). Modern practices to control the disease occurrence are application of nutrients and plant defense activators (Kaloshian and Walling, 2005). Systemic acquired resistance is an approach from potential plant disease management, which stimulates defense process in the host. It can be acquired by the usage of benzoic acid, citric acid, salicylic acid, K2HPO4 and KH2PO4 (Baloch et al., 2018). Salicylic acid is the most common inducer, which simulates the systemic actions of specific infection (Vallad and Goodman, 2004; Baloch et al., 2018). It also affects the plant growth and development, flowering, photosynthesis, ion uptake by a signal molecule and enzymatic activities (Uzunova and Popova, 2000). Similarly, proper application of macronutrients plays a vital role in improving plant health which generates resistance against disease. Nutrient deficient plants are more susceptible to disease, but their adequate amounts make a plant more tolerant or resistant against disease (Dordas, 2008). On the other hand, same nutrient in some environment may decrease the disease incidence but sometimes, it may increase incidence in other environment (Baloch et al., 2018). Mineral nutrition can produce antifungal compound in plants. Insect vectors and fungi are common sources to transmit these plant viruses. According to Oostendorp et al. (2001), silicon is not effective against sucking insects in order to reduce virus infection.
The main objective of this study was to identify the most effective concentration of nutrient or plant activator against potato leaf roll virus as an alternative pathway for effective disease management.
Materials and Methods
Collection of infected tubers
Infected tubers were collected from Plant Virology Section, Plant Pathology Research Institute, Ayub Agricultural Research Institute, Faisalabad. These tubers were taken from the infected plants of potato leaf roll virus (PLRV) which were confirmed through enzyme linked immunosorbent assay (ELISA) from crop of the previous season.
Establishment of experiment
To check the response of five plant defense activators i.e., salicylic acid, benzoic acid, dipotassium hydrogen phosphate (K2HPO4), potassium dihydrogen phosphate (KH2PO4) and citric acid against PLRV under field conditions, a field experiment was executed in RCBD with factorial fashion having three replications. Control was also kept for comparison. A potato advance line ‘FD69-1’ was sown during the years 2018 and 2019 at Virology Section, Plant Pathology Research Institute, AARI, Faisalabad. Three concentrations (0.25%, 0.50% and 0.75%) of each plant activator was prepared and sprayed three times on foliar part of plants with 15-day interval. In another experiment, treatments were comprised of solution of macronutrients (N, P and K) and micronutrients (B, Zn, Cu, Mn and Fe). These were also prepared and sprayed in aforementioned concentrations. The glass house experiment was executed in CRD. The pots were treated in the same manner with the regular intervals of 15 days. Control was also included to make comparison.
Data recording
Data were collected for disease management through plant activators, macronutrients and micronutrients. Disease incidence was calculated to compare the efficiency of treatments with the control. It was recorded on the basis of visual symptoms of every dose. Following formula was used to calculate the incidence percentage (Madden and Hughes., 1995).
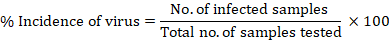
Data analysis
The trend was similar during both years. So, the data were averaged. After analysis of variance, differences among the treatment means were compared by applying Least Significant Difference test at 0.05 level of probability (Gomez and Gomez, 1984).
Results and Discussion
In first experiment, systemic resistance to manage Potato Leaf Roll Virus (PLRV) was induced through application of plant defense activators with three different concentrations. Different activators, concentrations of activators and their interaction had significant effects on PLRV (Table 1). Individually, salicylic acid showed significant control over PLRV (18.63%) as compared to other all other plant defence activators, while 0.75% concentration of the activators showed significant control over disease (35.65%) as compared to lower concentrations (Table 1). The interaction revealed that salicylic acid showed the most effective control over disease at 0.75% concentration (16.27%). It was followed by salicylic acid at concentration of 0.50% (19.39%) and 0.25% (20.25%), showing thereby that salicylic acid was the most effective activator sprayed at any concentration compared with all other activators.
Table 1: Effect of different concentrations of plant activators on incidence of Potato Leaf Roll Virus (2-year average).
|
Treatments |
Concentrations |
|||
|
0.25% |
0.50% |
0.75% |
Means |
|
|
Salicylic acid |
20.25 l |
19.39 l |
16.27 m |
18.63 f |
|
Benzoic acid |
27.33 i |
24.81 j |
21.41 k |
24.51 e |
|
KH2PO4 |
33.08 f |
31.57 g |
28.60 h |
31.08 d |
|
K2HPO4 |
39.78 c |
36.51 e |
33.43 f |
36.58 c |
|
Citric acid |
40.87 b |
38.66 d |
36.65 e |
38.73 b |
|
Control |
77.67 a |
77.78 a |
77.55 a |
77.67 a |
|
Means |
39.83 a |
38.12 b |
35.65 c |
|
|
LSD (activators)= 0.500; LSD (concentrations)= 0.354; LSD (interaction)=0.866 |
||||
In second experiment, systemic resistance to manage Potato Leaf Roll Virus (PLRV) was induced through foliar application of micronutrients and macronutrients with three different concentrations. Nutrients, concentrations of nutrients and their interaction showed significant effects on PLRV (Table 2). Individually, macronutrients showed significantly more control over PLRV (24.52%) than micronutrients (38.55%), while 0.75% concentration of nutrients showed significant control over disease (41.72%) as compared to lower concentrations (Table 2). The interaction revealed that foliar spray of macronutrients @ 0.75% resulted in the most significant reduction in disease incidence (21.50%). It was followed by application of macronutrients at concentration of 0.50% (24.50%) and 0.25% (27.56%), showing thereby that application of macronutrients was the more effective sprayed at any concentration than micronutrients.
Table 2: Effect of different concentrations of macro and micro-nutrients on Potato Leaf Roll Virus (2-years average).
|
Treatments |
Concentrations |
Means |
||
|
0.25% |
0.50% |
0.75% |
||
|
Macronutrients |
27.56 e |
24.50 f |
21.50 g |
24.52 c |
|
Micronutrients |
40.57 b |
38.53 c |
36.53 d |
38.55 b |
|
Control |
66.68 a |
66.56 a |
67.12 a |
66.79 a |
|
Means |
44.94 a |
43.19 b |
41.72 c |
43.28 |
|
LSD (nutrients)= 0.624; LSD (concentrations)= 0.624; LSD (interaction)= 1.081 |
||||
On an average, 78% Potato Leaf Roll Virus (PLRV) was observed in controlled plots. 76% disease was observed to be decreased by foliar application of salicylic acid compared with control. It was followed by benzoic acid (68%), KH2PO4 (60%), K2HPO4 (53%) and citric acid (50%). It revealed that all activators had potential to control PLRV with some variations. Furthermore, 6.48% more effectiveness of activators against PLRV was observed when sprayed at 0.75% than that of 0.50% concentration and 10.49% more than that of 0.25% concentration.
Defense response in plants can be produced by plant activators. A sustainable and effective control is achieved by the use of plant activators which reduce speed of multiplication of pathogen and produce resistance. On the other hand, some activators are also reported to boost up plant vigour and yield (Gullino et al., 2000). It is a powerful tool to stimulate the plant defense responses against insect-pest and diseases. For many decades, induced resistance study was done in laboratories. With the available reports, it has been found inconsistent for management of pests in field. The plant activators can be defined as plant defense-activating compounds. These plant defense activators are also known as systemic acquired resistance inducers and are also named as biopesticides. These activators have no direct influence on the disease-causing pathogens. In integrated pest management (IPM) program, plant activators could be a practicable component by delaying early fungicide and insecticide applications on it and these could be substituted with the chemical control. Di-potassium hydrogen phosphate (K2HPO4), salicylic acid (SA) and ferric chloride (FeCl3) were used as plant defense activators. Salicylic acid @ 3mM showed maximum decrease in PLRV (51.8%) as compared to control, reported by Ali (2016), while 76% decrease in disease by salicylic acid was observed in our study. Similarly, these results are almost supported by those of Mushtaq et el. (2019) who reported that salicylic acid performed best at 1.0% concentration against potato leaf blight disease as compared to control. It was followed by KH2PO4, benzoic acid, K2HPO4, citric acid and calcium chloride. External application of salicylic acid or other activators like citric acid, K2HPO4, benzoic acid and KH2PO4 produce the pathogenicity related proteins which reduce the incidence of diseases (Gozzo, 2003). Foliar application of salicylic acid on tomato infected with stem canker significantly lessened the disease index contrary to its application on other infected crops (Esmailzadeh et al., 2008). Hao et el. (2014) narrated that application of salicylic acid significantly increased the accumulation level of H2O2 in plant parts. It revealed systemic hypersensitive responses and acquired resistance against pathogens. The modern techniques of managing PLRV should be practiced as there is no chemical introduced in the market till now that can control the viral infection. So, the method of inducing the systemic acquired resistance through foliar application of plant activators like salicylic acid is an important technique of managing the disease.
In second experiment, 67% Potato Leaf Roll Virus (PLRV), on an average, was observed in controlled plots. 63% disease was observed to be decreased by foliar application of macronutrients and 42% by that of micronutrients solution compared with control. It revealed that both nutrient solutions had the potential to control PLRV. To get better results, both macro- and micro-nutrients should be sprayed in combination. Furthermore, nutrients sprayed at 0.75% concentration was observed 7.17% more effective against PLRV than 0.50% concentration and 3.42% more than 0.25% concentration.
Dordas (2008) observed the differences in the response of obligate parasitic pathogens to nitrogen supply. He observed that infection severity level increased as the level of N increased. On the other hand, when facultative parasite outbreaks and N-level got higher, the level of infection severity declined. The host susceptibility dropped down up to an optimal level for growth as the level of K diminished and by crossing this limit, there was no further rise in plant resistance level. The role of P was in contrast to K. It disclosed that the resistance was variable and nitrogen apparently inconsistent. He further noted that Zn was affecting to have a number of different effects on the development of diseases as in some cases, it caused decrease in level of infection while in others, it caused increase in infection, and in some cases, it had no effect on plant susceptibility to the disease. According to Dordas (2008), Mn has also a vital role in photosynthesis, lignin biosynthesis, phenol biosynthesis and many other vital functions. He found B reducing the severity of many diseases because of its effects on cell plant metabolism, wall structure and membrane development. Similarly, application of Cl can also improve the resistance of host plant to the disease. Study of Dordas (2008)showed that Si generated a physical barrier which confined the growth and hyphae penetration of fungus. He concluded that integrated plant nutrition was an important aspect in sustainable agriculture because it is an eco-friendly (curtain the use of passive approach) and cost-effective to manage plant diseases (Dordas, 2008). In nutshell, nutrients are effective to reduce disease incidence to a specific level and also boost up growth and vigour of plants. Plant activators and nutrients are safer, comparatively cheap and more successful as compared to conventional organic biopesticides.
Conclusions and Recommendations
Although there is no appropriate antiviral compound available in the market to control plant viruses but justified use of recent management techniques such as induction of resistance through spray of plant activators and nutrients are effective to manage Potato Leaf Roll Virus (PLRV). Although all activators (salicylic acid, benzoic acid, KH2PO4, K2HPO4, citric acid) were found effective to manage PLRV with some variations but salicylic acid with 0.75% concentration was found the most effective to manage PLRV as compared to others. Both nutrient solutions had potential to control PLRV but macronutrients (N, P, K) solution with 0.75% concentration were found more effective than micronutrients (B, Zn, Cu, Fe and Mn) solution to manage PLRV. It is advisable to manage PLRV, both macro- and micro-nutrients should be sprayed in combination to get better results.
Novelty Statement
The study focuses on capability of plant activators and nutrients to manage potato leaf roll virus.
Muhammad Musa: Conceived the idea and wrote the manuscript.
Saba Saeed: Conducted the research study and analyzed data.
Saima Naseer and Muhammad Ussama Yasin: Helped in compiling data.
Azhar Mustafa: Supervised the research study and did proof reading.
Iftikhar Haider: Edited the manuscript.
All authors read and approved final draft for publication.
Conflict of interest
The authors have declared no conflict of interest.
References
Abbas, A., M. Arif and A. Ali. 2016. Use of hot water-thermotherapy to free potato tubers of potato leaf roll virus (PLRV). Int. J. Life. Sci. Res., 2(2): 155-162.
Abbas, M.F., S. Hammed, A. Rauf, Q. Nosheen, A. Ghani, A. Qadir and S. Zakia. 2012. Incidence of six viruses in potato growing areas of Pakistan. Pak. J. Phytopath., 24(1): 44-47.
Ali, A.A., 2016. Screening of potato germplasm against potato leaf roll virus and its management through resistance inducer chemicals. M.Sc. thesis. Dep. Plant Pathol. Univ. Agric., Faisalabad, Pakistan. https://agris.fao.org/agris-search/search.do?recordID=PK2018000261
Ali, A., S. Hassan and A. Asad. 2002. Incidence of six potato viruses in spring, summer and autumn potato crops of the North West Frontier province of Pakistan. Aust. Plant Pathol., 31(2): 143-146. https://doi.org/10.1071/AP02006
Ali, H., R.A. Shah, R.Q. Zeb, H. Badshah and M. Rehaman. 2011. Evaluation of some chemicals against the aphids, jassids and white flies in potato. Sci. Int. (Lahore), 23: 67-69.
Anonymous, 2018. Potato leafroll virus in potato crops. Agriculture and Food, Department of Primary Industries and Regional Development. Government of Western Austrialia.
FAOSTAT, 2020. Food and agricultural organization crops. http://www.fao.org/faostat/en/#data/QC
Baloch, M.S., N.A. Rajput, M. Atiq, A. Rehman, S.M. Khan, K. Naveed, B. Khan, S. Ullah and N.M. Baloch. 2018. Effect of different plant activators against Rhizoctonia solani causing root rot of chilli. Pak. J. Phytopath., 30(1): 45-51. https://doi.org/10.33866/phytopathol.030.01.0441
Bhutta, A., and M. Bhatti. 2002. Seed potato certification in Pakistan. Federal Seed Certification and Registration Department, Ministry of Food Agriculture and Livestock, Islamabad. Pakistan. pp. 60-66.
Dordas, C., 2008. Role of nutrients in controlling plant diseases in sustainable agriculture: A review. Agron. Sustain. Dev., 28(1): 33-46. https://doi.org/10.1051/agro:2007051
Esmailzadeh, M., M. Soleimani and H. Rouhani. 2008. Exogenous applications of salicylic acid for inducing systemic acquired resistance against tomato stem canker disease. J. Biol. Sci., 8(6): 1039-1044. https://doi.org/10.3923/jbs.2008.1039.1044
Gebhardt, C. and J.P. Valkonen. 2001. Organization of genes controlling disease resistance in the potato genome. Ann. Rev. Phytopathol., 39(1): 79-102. https://doi.org/10.1146/annurev.phyto.39.1.79
Gomez, K.A. and A.A. Gomez. 1984. Statistical procedures for agricultural research. New York: John Wiley and Sons.
Gozzo, F., 2003. Systemic acquired resistance in crop protection: from nature to a chemical approach. J. Agric. Food Chem., 51(16): 4487-4503. https://doi.org/10.1021/jf030025s
Gullino, M.L., P. Leroux, and C.M. Smith. 2000. Uses and challenges of novel compounds for plant disease control. Crop Protect., 19(1): 1-11. https://doi.org/10.1016/S0261-2194(99)00095-2
Hao, W., H. Guo, J. Zhang, G. Hu, Y. Yao and J. Dong. 2014. Hydrogen peroxide is involved in salicylic acid-elicited rosmarinic acid production in Salvia miltiorrhiza cell cultures. Sci. Res. J. Special Issue, https://doi.org/10.1155/2014/843764
Kaloshian, I., and L.L. Walling. 2005. Hemipterans as plant pathogens. Ann. Rev. Phytopathol., 43(1): 491-521. https://doi.org/10.1146/annurev.phyto.43.040204.135944
Khalid, F., A. Mubarik and Y. Aqsa. 2020. Potato cluster feasibility and transformation study. In: A. Mubarik, (ed.). Cluster development based agriculture transformation plan vision-2025. Planning Commission of Pakistan, Islamabad, Pakistan and Centre for Agri. and Biosci. Inter. (CABI), Rawalpindi, Pakistan. https://www.pc.gov.pk/uploads/report/Potato_Cluster_Report.pdf.
Madden, L.V. and G. Hughes. 1995. Plant disease incidence: Distributions, heterogeneity, and temporal analysis. Ann. Rev. Phytopathol., 33(1): 529-564. https://doi.org/10.1146/annurev.py.33.090195.002525
Mughal, S., 2003. Some threatening and emerging plant viral diseases in Pakistan. Proc. 4th Nat. Conf. Plant Pathol. Held on 14-16 Oct. pp. 8-16.
Mushtaq, M.S., N.A. Rajput, M. Atiq, A.M. Lodhi, B. Khan, A. Hameed, S. Sarfraz, N. Muhammad, G.A. Kachelo and H. Firdos. 2019. Activation of potato defence system against leaf blight disease through plant activators. Fresenius Environ. Bull., 28(12A): 9716-9723.
Oerke, E.C., 2006. Crop losses to pests. J. Agric. Sci., 144(1): 31-43. https://doi.org/10.1017/S0021859605005708
Oostendorp, M., W. Kunz, B. Dietrich and T. Staub. 2001. Induced disease resistance in plants by chemicals. Eur. J. Plant Pathol., 107(1): 19-28. https://doi.org/10.1023/A:1008760518772
Saljoqi, A.U.R., 2009. Population dynamics of Myzus persicae (Sulzer) and its associated natural enemies in spring potato crop, Peshawar, Pakistan. Sarhad J. Agric., 25(3): 451-456.
Uzunova, A. and L. Popova. 2000. Effect of salicylic acid on leaf anatomy and chloroplast ultrastructure of barley plants. Photosynthetica, 38(2): 243-250. https://doi.org/10.1023/A:1007226116925
Vallad, G.E. and R.M. Goodman. 2004. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci., 44(6): 1920-1934. https://doi.org/10.2135/cropsci2004.1920
To share on other social networks, click on any share button. What are these?






