Utilization of Emulsiflex Prepared Escherichia coli Strain EC4 Oxyrase for Improved Cultivation of Anaerobic Bacteria by using Hungate Technique and Determination of its Kinetic Parameters
Utilization of Emulsiflex Prepared Escherichia coli Strain EC4 Oxyrase for Improved Cultivation of Anaerobic Bacteria by using Hungate Technique and Determination of its Kinetic Parameters
Muhammad Usman Ahmad and Ikram-ul-Haq*
Institute of Industrial Biotechnology, GC University, Lahore-54000
ABSTRACT
Present study was conducted for the utilization of Emulsiflex prepared oxyrase extracted from cytoplasmic of membrane fragments of E. coli. For this purpose, 88 E.coli isolates from 30 intact poultry intestines were cultured and identified preliminary on the basis of microscopy and biochemical testing. E. coli strain EC4 was screened as best oxyrase producer with activity of 0.41±0.008U/mL/min with 41% reduction in dissolved oxygen at pH 7.5, temperature 37°C, 25mM lactate as H+ donor after 20 min. This strain was later on confirmed by 16S ribotyping procedure. Oxyrase was used for improved cultivation of anaerobic bacteria by employing Hungate Technique where its antioxidant potential was compared with common reducing agent Cysteine-HCl (Cys-HCl). Four anaerobic bacterial strains (Anaerobaculum hydrogeniformans OS1, Akkermansia muciniphila, Bilophila wadsworthia, and Roseburia intestinalis) were selected as experimental models for this purpose. Significant improvement in cell density of Anaerobaculum hydrogeniformans culture with maximum OD600nm of 0.80±0.0017 reached after 4 days of incubation when oxyrase used as reducing agent, and maximum OD600nm 0.65±0.0016 in presence of Cys-HCl. Akkermansia muciniphila culture reached maximum OD600nm value of 2.1±0.07 in the presence of oxyrase after 18 h of incubation. While with Cys-HCl, Akkermansia muciniphila culture reached maximum cell density OD600nm of 2.0±0.05 after 27 h of incubation. Oxygen reducing potential of oxyrase also suited the growth of Bilophila wadsworthia because it reached maximum cell density OD600nm of 2.0±0.05 after 73 h of incubation. Whereas, in the presence of Cys-HCl Bilophila wadsworthia cells only achieved maximum OD600nm of 1.54±0.07 after 28 h of incubation. Cultivation studies of Roseburia intestinalis in the presence of oxyrase also exhibited noteworthy improvement in the yield of cell density with OD600nm of 2.1±0.06 after 25 h of incubation. With Cys-HCl, maximum cell density of Roseburia intestinalis with 2.0±0.06 OD600nm was observed after 73 h. Kinetic parameters derived from double reciprocal Lineweaver-Burk plot showed Km values 8.49×10-3 M-1 and 4.75×10-3 M-1 of sonication and Emulsiflex prepared oxyrase membrane fragments, respectively for lactate as substrate.
Article Information
Received 29 June 2018
Revised 10 August 2018
Accepted 31 October 2018
Available online 15 March 2019
Authors’ Contribution
MUA conceived, designed and performed the experimental work. MUA and IUH prepared the manuscript. IUH supervised the experimental work.
Key words
Oxyrase, Escherichia coli strain EC4, Hungate technique, Cytoplasmic membrane fragments and Emulsiflex.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.3.835.834
* Corresponding author: ikmhaq@yahoo.com
0030-9923/2019/0003-0825 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Certain enzyme systems in cytoplasmic membrane fragments of some bacteria can efficiently catalyze the enzymatic reduction of dissolved oxygen (DO) in medium to H2O. Recent studies have drawn attention towards practical applications of these membrane fragments for reduction of DO to water by a terminal chain reaction in electron transport system of aerobic respiration (Palmer and Bonner, 2007). Various studies have reported several microorganisms for production of oxygen reducing cytoplasmic membrane fragments, such as Escherichia coli, Gluconobacter oxydans, Pseudomonas aeruginosa, Mycobacterium phlei, Azotobacter vinlandii and Salmonella typhimurium (Ahmad et al., 2017; Adler, 1990).
E. coli is a gram-negative, rod-shaped, facultative anaerobic, non-spore forming bacterium which sometimes may form a capsule. Basic biochemical characteristics of this Enterobacterium are oxidase negative and catalase positive, as well as ability to ferment glucose and reduction of nitrates to nitrites. E. coli is a common inhabitant of poultry intestinal tracts at numbers up to 106/g of fecal material. Birds without an established microflora, younger birds, and lower intestinal tract reportedly possess higher densities of E. coli (Jin et al., 1997). Poultry waste is regarded as a rich source of bacterial flora with ability to express array of enzymes (Rehman and Imtiaz, 2018).
Ribotyping is one of the frequently used methods to study taxonomy and bacterial phylogeny. It utilizes 16S rRNA gene sequences as most common genetic marker for various reasons, such as: its gene size of 1500 bp is large enough for informatics analysis; non-redundancy of its function; its highly conservative existence as multigene family or operons in almost all bacteria (Janda and Abbott, 2007).
Avestin Emulsiflex-C3® is very efficient, with capacity of 3L/h, and easy to use for efficient E. coli cell lysis in one passage (with 90% efficiency) at 100 MPa (15,000 psi) in laboratory setups (Andrews and Asenjo, 1987). Concomitant decrease of redox potential from 200 to 300 mV by utilizing oxygen reducing membrane fragments to complex microbiological media has been reported. Without utilizing any other chemical reducing agent, small amounts of sterile E. coli cytoplasmic membranes are being added to bacteriological media for cultivation of anaerobic bacterial strains. These preparations comprising of E. coli membrane fragments can reduce DO in few minutes after they were added to create microanaerboic environments. By any means during incubation, diffusing oxygen in medium from the head space gets rapidly reduced to water and hence keeping it anaerobic (Adler and Spady, 1997).
DO can be detected in the medium by various methods like electrochemical (potentiometric, amperometric, or conductometric), chemical (Winkler) and pptical (photoluminescence). But since the development of Clark Electrode/Probe based method, it has been widely used for measurement of DO because of its reliability and sensitivity (Quaranta et al., 2012).
Obligate anaerobes are those microorganisms which are not able to utilize molecular oxygen for their growth. Hungate technique is one of the recommended methods for culturing of anaerobic bacteria in research laboratories. Anaerobaculum hydrogeniformans is a moderately thermophilic, anaerobic, NaCl-requiring, Gram-negative, non-spore forming, fermentative, rod-shaped bacterium. Strain OS1 has been originally isolated from oil production water reserves of Alaska, USA (Maune and Tanner, 2012). Akkermansia muciniphila is an oval-shaped, non-motile, non-spore-forming, gram-negative, and strictly anaerobic bacterium (Dao et al., 2016). Bilophila wadsworthia is recognized as obligate anaerobic, nonspore forming, rod shaped gram-negative bacterium (Sawamura et al., 1997). Roseburia intestinalis is gram-positive, anaerobic, slightly curved rod shaped bacterium and exhibit motility by multiple sub-terminal flagella (Duncan, 2002).
Present work was aimed at utilizing Emulsiflex for the first time to prepare oxygen reducing cytoplasmic membrane fragments from Escherichia coli strain EC4. Immense antioxidant potential of oxyrase enzyme system in these membrane fragments was evaluated. Combination of oxyrase as reducing agent and the Hungate technique for cultivation of anaerobic bacteria was used for this purpose.
Materials and methods
Isolation, preliminary identification and screening of oxyrase producing E. coli
Thirty intact poultry intestines were collected in a clean decontaminated polyethylene zipper bag, from 30 sites/broiler poultry butcher shops located in three different areas: Ichhra, Anarkali and Rehmanpura Lahore. Approximately three inches of distal colon at first point proximal to rectum, which contained feces, was resected with sterile scalpel and all fecal material was aseptically transferred to 10mL sterile phosphate-buffered saline (pH 7.4) in test tubes (Feder et al., 2003). Fecal material in test tubes was mixed/homogenized by repeated pipetting.
Eosin methylene blue (EMB) agar medium plates were inoculated with 100µL diluted samples by spread plate method on and incubated at 37°C for 24 h. Quadrant streak plate method was used for pure culturing of E. coli. All different isolates from various samples were separately maintained on nutrient agar (pH 6.8) slants for further identification.
Loop smears of isolates were prepared from E. coli characteristic metallic-sheen green colonies on EMB agar master plates. Gram staining of smears was performed according to Cheesbrough (2006). Microscopic observations of stained smears were done at 100X objective using OLYMPUS® CH30 BINOCULAR Microscope. Various recommended biochemical tests were performed for preliminary identification of isolates as E. coli (Holt et al., 1994; Cheesbrough, 2006). Twenty four hours old cultures of isolates were used for biochemical identification. Upstream, downstream process for screening of oxyrase for E. coli isolates was setup according to methodology described in our previous work (Ahmad et al., 2017).
Quantitative determination of oxyrase units
Benchtop dissolved oxygen meter was used for quantification of oxyrase according to manufacturer’s protocol. Reaction suspension with total volume of 5.0mL was constituted containing 500µL of membrane preparation + 4.5mL of phosphate buffer (pH 7.5) was incubated at 37°C for 3 min. 10mM of H+ donors was formulated in phosphate buffer to start the reaction. Then probe was submerged in sample, occasionally stirred gently and measured value (in %) was recorded once the reading was stabilized. End point reading was noted after 20 min (Tuitemwong et al., 1994).
One oxyrase unit is defined as ability of integrated enzyme system in sterile cytoplasmic membrane fragments of E. coli to reduce 1% dissolved oxygen per mL of solution in one minute at 57°C, pH 8.5. Following equation was derived from Wongjaroen et al. (2008), and used to calculate oxyrase activity (OA):
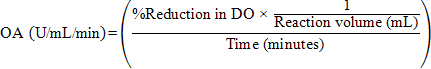
Molecular confirmation of best E. coli isolate with maximum oxyrase activity
Ribotyping of the strain was performed for molecular confirmation. Extraction of genomic DNA of E. coli was performed with Thermo Scientific® GeneJET Genomic DNA (gDNA) Purification Kit# K0721 by using manufacturer’s protocol. Conventional PCR was set up with specific universal primers for E. coli: 27F and 1492R (Frank et al., 2008). Total PCR reaction mixture (25 µL) comprised: 12.5 µL Thermo Scientific® PCR Master Mix (2X) # K0171+0.5 µL of 10 µM each primer+2 µL E. coli gDNA, and rest of final volume was raised with deionized water.
PCR was set up in thermocycler (Biometra®) by initial denaturation at 94°C for 5 min followed by 30 cycles, each of denaturation at 94°C for 30 sec, annealing at 54°C for 30 sec and extension at 72°C for 2 min. Final extension was done at 7°C for 10 min. PCR product was purified with Thermo Scientific® GeneJET Gel Extraction and DNA Cleanup Micro Kit#K0831 by using manufacturer’s protocol. Purified PCR product was run on 1% agarose gel (EtBr added in 0.5 µg/mL final concentrations). Thermo Scientific® GeneRuler DNA Ladder Mix, ready-to-use, # SM0334 (100-10,000 bp) was used as standard. BIO-RAD® agarose gel electrophoresis horizontal apparatus was used. Gels were allowed to run at 100V for 35 min.
PCR cleaned product containing amplified 16SrRNA gene from E. coli isolate was sent to Applied Biosciences International, Malaysia for sequencing. The 16SrRNA sequence was then aligned with representative 16S rRNA sequences belonging to related taxa with Clustal Omega online tool of EMBL-EBI (McWilliam et al., 2013). By using neighbor-joining method (Saitou and Nei, 1987) and Jukes and Cantor model (Jukes and Cantor, 1969) of software MEGA 6.0 (Tamura et al., 2013), phylogenetic tree was generated.
Use of Avestin Emulsiflex-C3® for preparation of oxyrase containing cytoplasmic membrane fragments
E. coli inoculum (30mL) was raised overnight in LB broth medium. Modified RF medium consists of peptone, 3.0 g/L; yeast extract, 2.5 g/L; KH2PO4, 2.5 g/L; MgSO4.7H2O, 0.2 g/L; glucose, 15.0 g/L and ammonium sulphate (NH4(SO4)2), 2.0 g/L (Rattray and Fox, 1997) was formulated and dispensed separately in 1L volume in three 2.5L Thomson™ Ultra Yield™ flasks. Flask’s necks were covered by aluminium foil and sterilized by autoclaving. 10mL inoculum was aseptically added to each flask, and incubated for 16 h at 37°C, 180 rpm in New Brunswick Scientific™ Innova® 44 Incubator Shaker.
Culture broth was centrifuged at 4000rpm for 20 min at 4°C to pellet the cells. Cell pellet (1.0 g) was resuspended in 3mL of buffer (50mM Tris pH 8.0 + 300mM NaCl) by vortexing. EDTA (1mM final concentration) + 200µL benzoase from 2mg/mL stock in 50% glycerol + PMSF (phenylmethylsulfonyl fluoride) in final concentration of 0.01% was added to resuspended pellet and mixed by pipetting.
Resuspended cell pellet and buffer bottles were preceded on crushed ice for cell lysis by using Avestin Emulsiflex-C3® homogenizer. Cell lysis was performed according to standard protocol (Tong, 2011) and was harvested in a clean bottle placed on crushed ice. MgCl2 (10mM final concentration) was added later on after cell disruption, and mixed by pipetting.
Disrupted/lysed cell sample was centrifuged at 17000rpm, 4°C for 30 min in Beckman fixed angle JA-17 rotor using Beckman Coulter® high speed floor centrifuge machine to pellet cell debris. Supernatant with membrane fragments were harvested and sterilized by using Corning® 500mL vacuum filter system, 0.22µm pore, 33.2cm² sterile polyethersulfone (PES) membrane for further analysis (Standard Operating Procedures v2.4 developed by Protein Expression Technology Center, Paul D. Boyer Hall UC Los Angeles DOE).
Characterization of oxyrase activity of emulsiflex prepared purified membrane fragments from E. coli
Effect of time (0-150 seconds) on oxygen scavenging potential of Emulsiflex mediated membrane preparation was evaluated started with 100% oxygen saturation. Characterization of emulsiflex mediated prepared oxyrase activity was also performed at 57°C and pH 8.5 as optimized in our previous work (Ahmad et al., 2017) in presence of various concentrations (0-50mM) of Formate, Lactate, α-glycerophosphate and Succinate as H+ donors/substrate.
Utilization of Avestin Emulsiflex-C3® prepared E. coli oxyrase containing cytoplasmic membrane fragments for improved cultivation of anaerobic bacteria
Pure and identified strains of four anaerobic test bacteria: Anaerobaculum hydrogeniformans strain OS1, Akkermansia muciniphila, Bilophila wadsworthia and Roseburia intestinalis were taken from culture stock of Department of Microbiology, Immunology and Molecular Genetics, University of California Los Angeles. Anaerobic cultivation of these strains for in vitro evaluation of oxyrase by using Hungate Technique were setup according to protocols originally described by Hungate (1969) and Bryant (1972). Scheme for anaerobic media composition was provided by Prof. Dr. Robert P. Gunsalus (MIMG, UCLA). Detailed media composition for all four anaerobic bacteria used is provided in supplementary material file.
In one set of three tubes (for triplicate readings), 0.1mL of 2.5% Na2S-Cys-HCl stock was aseptically added. In other set of three tubes (for triplicate readings), 0.6mL of oxyrase buffer reagent-without methylene blue (Ahmad et al., 2017) + 0.4mL of oxyrase preparation was aseptically added. Experimental/culture tubes were inoculated with 2% inoculum (0.6 OD600nm). Uninoculated tubes for both Na2S-Cys-HCl and oxyrase (as reducing agents) were separately set as blank for spectrophotometric analysis of growth. Biochrom® WPA CO8000™ Cell density meter, with test tube sample containers, was used for analysis of OD values at 600nm. Prepared media were autoclaved at 121°C (liquid cycle) for 30 min with slow exhaust cycle.
Determination of kinetic parameters of oxyrase
Double reciprocal Lineweaver-Burl plot was used to estimate Km (Michaelis-Menten constant) and Vmax (maximum reaction velocity) values of oxyrase for all substrates as described by Lineweaver and Burk (1934). Graph was plotted between reciprocal of V (reaction velocity, calculated according to equation A) and reciprocal of used substrates. Equation of straight line from plot was referred to Lineweaver-Burk equation (Equation B) to calculate kinetic parameters (Bennett and Frieden, 1969). Equations C and D were used to calculate Vmax and Km values.

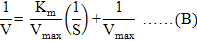
Whereas,
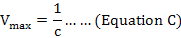

Data analysis
For processing the data, statistical methods given by Snedecor and Cochran (1980) was adopted by using Costat Computer software. Experimental means were compared by Completely Randomized One Way ANOVA (Analysis of Variance). Duncan’s Multiple Range Test was used for this purpose with significance level of 5%.
Results and discussion
Present study was conducted for utilization of E. coli cytoplasmic membrane fragments’ electron transport chain composite enzyme system hereby referred as oxyrase. This enzyme system is responsible for reduction in dissolved oxygen from the medium it has been employed and is very suitable for generation of microanaerboic environments for cultivation of anaerobic microorganisms. In our best knowledge, present work is first of its kind in which Emulsiflex is used for preparation of oxyrase, and Hungate technique was employed for evaluation of its antioxidant potential.
For the purpose of isolation of E. coli, poultry intestine samples because of its heavy colonization with the bacterium. Out of 30 intact poultry intestine samples; 08 samples were collected from site 1 (Anarkali) resulted in 28 isolates of E. coli (EC1-EC28); 19 isolates (EC29-EC47) and 41 isolates (EC48-88) were found in 7 samples from site 2 (Rehmanpura) and 15 samples from site 2 (Ichhra), respectively. E. coli isolate showed differential appearance of characteristic metallic sheen colonies of E. coli on EMB agar medium as reported by Cheesbrough (2006). This result is in accordance to Jin et al. (1997) as they have also reported E. coli isolation with its abundant distribution in duodenum and jeju-ileum of chicken. Frequent isolation of large number of E. coli strains from poultry intestines in present work is in accordance with Carson et al. (2001). They showed similar kind of results reported for identification of abundant fecal E. coli isolates from humans and animals. Furthermore, they also reported heavy inhabitation of E. coli in poultry intestinal tracts.
These 88 (numbered in series as EC1-EC88) were pink red Gram negative straight rods. They gave negative oxidase, citrate utilization and gelatin hydrolysis test and positive catalase carbohydrate fermentation and methyl red tests. These results in accordance to Holt et al. (1994) and Cheesbrough (2006) inferred their identification as E. coli. EC4 was the best strain in terms of oxygen reduction ability, with activity of 0.41±0.008 U/mL/min which dissipated 41% of dissolved oxygen at standard defined conditions.
Frequency of active oxyrase by probe method suggested 76 isolates were positive for oxygen reduction potential. 37% of positive isolates showed activity range from 0.25-0.34 U/mL/min, 20% isolates with 0.15-0.24 U/mL/min, 22% isolates with 0.05-0.14 U/mL/min and 20% isolates’ activity ranged ≤0.04U/mL/min. This distribution of frequency in activity is in accordance along with variation to Wongjaroen et al. (2008). Variation in results of present and their study is due to difference in enzyme assay reaction mixture; and they also did not reported source habitat from where E. coli was isolated.
On the basis of screening results, E. coli oxyrase strain (EC4) was selected and further preceded for confirmation by ribotyping. PCR product with 1.5kB size 16SrRNA gene sequence was used for molecular confirmation of E. coli oxyrase strain. PCR with universal primers: 27F and 1492R, specific for E. coli was employed and agarose gel electrophoresis results showed specific amplification of gene size 1.5kB. This result is in accordance with Frank et al. (2008). Sequencing results were further analyzed by using online BLASTN 2.7.1+ tool of NCBI. Multiple sequence alignment was performed with online MUSCLE tool of EMBL-EBI. Phylogenetic tree constructed with MEGA 6.0 showed significant evolutionary relationship with 11 of E. coli strains with closest being E. coli strain W4 and E. coli strain KCJ5233 (Fig. 1). Carson et al. (2001) also reported confirmation of abundant E. coli by ribotyping, isolated from animal fecal samples. Ribotyping and Biochemical profiling confirmed EC4 strain as E. coli which was further used in rest of experimental work. The sequence was submitted to GenBank of NCBI with accession no. MH281943.1 and can be accessed online at https://www.ncbi.nlm.nih.gov/nuccore/MH281943.
Characterization of these purified Emulsiflex prepared membrane fractions were performed for quantification of active oxyrase; which revealed 100% reduction in dissolved oxygen at 57°C, pH 8.5, in presence of 25mM lactate as H+ donor, after 1.5 min with activity of 20±0.49 U/mL and specific activity of 8.04 U/mg (Figs. 2, 3). Maximum activity of 13.33±0.19 U/mL/min was observed at 25mM lactate. Increase in proton donor concentration resulted increase in oxygen reduction potential of membrane fragments. But at higher concentrations (≥25mM lactate) stable trend in oxyrase activity was observed. Response of Emulsiflex prepared oxyrase membrane fragments towards all substrates in various concentrations was found same as of sonication prepared fragments as reported in our previous work (Ahmad et al., 2017).
In presence of formate (25mM), 93.75% reduction in dissolved oxygen was observed, whereas α-glycerophosphate and succinate (25mM concentration) caused 81% and 70.5% reduction in DO, respectively. These results are in accordance to Tuitemwong et al. (1994) as they reported 100% depletion in oxygen in presence of lactate within 2 min. Therefore, this activity of oxyrase is 95% improved per unit time as compared to sonication mediated cell lysis for oxyrase production from E. coli in classical Erlenmeyer shake flask experiments. This dramatic improvement of oxygen reducing potential of Emulsiflex prepared E. coli membrane fragments over sonication prepared is due to obvious efficiency of Emulsiflex to cause cell lysis and keeping integral enzyme system intact in membranes (Andrews and Asenjo, 1987).
Highly improved oxyrase efficiency of Emulsiflex prepared membrane fragments can also be justified due to use of THOMSON™ ULTRA YIELD™ shake flasks in present work. These findings are in accordance to Ukkonen et al. (2011) and Brodsky and Cronin (2006). Moreover, regulated and improved protein production by E. coli using Emulsiflex is also reported by Schlegel et al. (2013), Savage et al. (2007) and Hintz et al. (2001). But use of THOMSON™ ULTRA YIELD™ and Avestin Emulsiflex-C3® Homogenizer for production of oxyrase form E. coli is first of its kind in present study.
Downstreaming of oxyrase membrane fragments from E. coli in presence of buffer, EDTA, benzoase, and PMSF also played vital role in improved activity of oxyrase in present study. E. coli cell pellet was resuspended in buffer constituted by mixing 50mM Tris pH 8.0 and 300mM NaCl. This buffer provided buffered slight alkaline and salt environment to biological membranes which dissociates extrinsic peripheral membrane proteins by disrupting hydrogen and electrostatic bonds. Membrane fragments constituted oxyrase activity are integral proteins extended in E. coli lipid bilayer. Therefore, the said buffer provided better physiological and biochemical environment to membrane preparations for better yield in Emulsiflex (Ohlendieck, 2004). EDTA is chelating agent and it chelated all Mg++ and Ca++ ions from outer LPS membrane in E. coli, therefore destabilized it and makes cell lysis easy to expose cytoplasmic membrane fragments better (EMBL protein purification protocol). Benzoase nuclease degraded all form of cellular DNA and RNA released from lysed cell. PMSF is a serine protease inhibitor and its addition in cell Lysate avoided protein loss by degradation (James, 1978). MgCl2 (10mM) was added after cell lysis to neutralize further effect of any residual EDTA left in cell Lysate.
In vitro evaluation of oxyrase in cytoplasmic membrane fragments of E. coli strain EC4 was investigated for its active antioxidant by using the Hungate technique. In first experiment, effect of E. coli oxyrase and its comparison to Cys-HCl was evaluated on growth of a moderately thermophilic and anaerobic bacterium Anaerobaculum hydrogeniformans OS1. Bacterium reached maximum OD600 of 0.65±0.016 with Cys-HCl, and 0.80±0.017 with oxyrase as reducing agent at 55°C after 4 days of incubation (Fig. 4A). This is a clear evidence of efficient oxygen reducing ability of oxyrase comparative to Cys-HCl, as oxyrase supplemented culture tubes reached 19% more cell density as compared to oxyrase. It is also obvious that during late growth phase, Anaerobaculum hydrogeniformans OS1 cells maintained higher OD600 value of 0.59±0.014 with oxyrase and 0.25±0.018 at day 15 of incubation. This is due to slow reduction ability of Cys-HCl and its reaction with dissolved oxygen results in production of chemical residues that proved toxic for bacterial growth (Maune and Tanner, 2012).
Efficiency of E. coli oxyrase was evaluated with growth of strictly anaerobic bacterium Akkermansia municiphila in second experiment. After 18 h of incubation at 37°C, Akkermansia municiphila cells reached maximum OD600 of 2.10±0.07 with oxyrase and OD600 of 1.66±0.05 with Cys-HCl. As expected, oxyrase worked with efficiency of 21% improvement in cell growth compared to Cys-HCl. Wheras, Cys-HCl tubes supplemented culture tubes reached maximum cell density with OD600 2.00±0.05 after 27 h of incubation. Furthermore, it is also very obvious from growth curve (Fig. 4B) that in late growth phase from time 18-24 h of incubation, Akkermansia municiphila cells density remained stable with oxyrase. During the same time of growth with Cys-HCl, the OD600 value did rise from initial value 1.66±0.05 to 2.00±0.05.
This observation clearly showed sustainable efficiency of oxyrase membrane fragments to reduce dissolved oxygen efficiently and quickly as compared to Cys-HCl. During last three hours of growth phase, cell density with oxyrase decreased to 1.90±0.05 due to obvious reason of decline/death phase of growth curve initiated at that point. Use of oxyrase for growth of Akkermansia municiphila and its comparison with Cys-HCl using Hungate technique is not reported before. However, Gehring et al. (2014) reported efficient recovery of another facultative anaerobic, mesophile bacterium L. monocytogenes from food samples, with commercially available EC-oxyrase.
A third experiment was conducted to evaluate efficiency of E. coli oxyrase on an obligate anaerobic clinically relevant bacterium Bilophila wadsworthia. This bacterium is reported to cause intra-abdominal abscesses (Feng et al., 2017; Baron et al., 1992). During initial growth phase of 8 hours, cell density of Bilophila wadsworthia remained almost same: 0.33±0.03 with Cys-HCl and 0.35±0.04 with E. coli oxyrase as reducing agents. Bacterium reached maximum OD600 of 2.00±0.05 in presence of oxyrase and 1.35±0.04 with Cys-HCl after 73 h of incubation, indicating 33% improvement in cell density with oxyrase (Fig. 4C). Another significant difference is that after 28 h of incubation, Bilophila wadsworthia cell density was 1.54±0.07 with Cys-HCl, which later on entered in decline phase and cell density started decreasing. This is due to generation of toxic chemical residues by Cys-HCl in culture tubes during late growth phase. However, with oxyrase the cell density not only remained stable during this phase of growth but a rise in OD600 of Bilophila wadsworthia cells was observed.
Use of Hungate technique with E. coli oxyrase for Bilophila wadsworthia is not reported before and it’s first of its kind in present study. Results showed presence of oxyrase supported growth of Akkermansia muciniphila much better as compared to Cys-HCl. Growth was improved by 20% in presence of oxyrase and maximum OD600nm of 2.1±0.07 was achieved in tubes with oxyrase after 18 h of incubation showing it efficiency to remove dissolved oxygen (Fig. 4D). However, Wongjaroen et al. (2008) evaluated membrane fragments extracted from E. coli on growth enteric, microaerophilic pathogenic bacterium Campylobacter jejuni. They also reported improved cultivation of Campylobacter jejuni in increased amount of E. coli membrane fragments in Mueller Hinton Broth medium without chemical reducing agents.
Figure 5 shows Lineweaver-Burk plot for Emulsiflex prepared oxyrase characterized at standard conditions. Present study is first of its kind for determination of kinetic parameters of oxyrase. Minimum Km value of 4.75×10-3 M-1 was observed with lactate as H+/substrate which defined maximum affinity of oxyrase membrane fragments with lactate for oxygen reducing potential. Second best affinity of membrane fragments was found for formate with Km value of 1.34×10-2 M-1. α-glycerophosphate and succinate showed weak affinities out of all used substrates with Km values of 1.76×10-2 M-1 and 2.13×10-2 M-1, respectively. Detail of kinetic parameters is shown in Table I.
Table I.- Comparative kinetic parameters of Emulsiflex prepared E. coli strain EC4 derived oxyrase
|
Substrate |
Vmax |
Km (M-1) |
Vmax / Km |
|
Formate |
142.85 |
1.34×10-2 |
1.06×104 |
|
Lactate |
125 |
4.75×10-3 |
2.63×104 |
|
α-glycerophosphate |
142.85 |
1.76×10-2 |
8.13×103 |
|
Succinate |
125 |
2.13×10-2 |
5.88×103 |
Conclusion
Oxyrase is a composite enzyme in Escherichia coli cytoplasmic membrane fragments which comprises synergistic activity of dehydrogenases and terminal reductases/oxidases in E. coli membranes as part of its electron transport chain. Oxyrase bearing membrane fragments were prepared Avestin Emulsiflex-C3® mediated lysis of local E. coli strain EC4 cells, isolated from poultry intestinal sample. Avestin Emulsiflex-C3® is used for first time for preparation of oxyrase in present work. These membrane fractions showed enhanced oxyrase activity with 13.33±0.19 U/mL/min and specific activity of 8.0 U/mg which caused 100% reduction in dissolved oxygen at 57°C, pH 8.5 after 1.5 min. Kinetic parameters also supported evidenced of strong substrate affinity by Emulsiflex mediated oxyrase membrane preparation. Hungate technique was employed for anaerobic cultivation of organisms and antioxidant potential of oxyrase was compared with commonly used dissolved oxygen scavenger Cys-HCl. Oxyrase outcompeted Cys-HCl and efficiently supported improved growth rates of tested anaerobic strains. This strategy for production and evaluation of oxyrase is hereby first time used in present work. Findings of present research work will prove bench mark for developing better facility of cultivation of anaerobic bacteria either for diagnostics or research purpose. As a specialized facility for cultivation of anaerobes is not available in Pakistan, use of oxyrase will replace inefficient and cumbersome approaches to achieve anaerobiosis and to use air tight anaerobic chambers to separately support growth of anaerobic bacterium.
Acknowledgements
Principle author is highly grateful to Prof. Dr. Robert P. Gunsalus, Dr. Mark Arbing, Farzaneh Sedighian, Sum Chan, Leo Liu and all the staff members of University of California, Los Angeles (UCLA) for all the scientific and technical support to conduct this research work. Authors also highly appreciate IRSIP team of Higher Education Commission of Pakistan, Islamabad for providing necessary funds for scholarship program to visit UCLA to conduct this research work.
There is supplementary material associated with this article. Access the material online at: http://dx.doi.org/10.17582/journal.pjz/2019.51.3.835.834
Statement of conflict of interest
The authors declare no conflict of interest.
References
Ahmad, M.U., Qamar, H., Anwar, Y., Javed, M.M., Babar, M.E. and Haq, I.U., 2017. Exploration and screening of oxygen reducing potential in cytoplasmic fragments of Escherichia coli and Salmonella sp. J. Anim. Pl. Sci., 27: 302-308.
Andrews, B.A. and Asenjo, J.A., 1987. Enzymatic lysis and disruption of microbial cells- A review. Trends Biotechnol., 5: 273-277. https://doi.org/10.1016/0167-7799(87)90058-8
Adler, H. and Spady, G., 1997. The use of microbial membranes to achieve anaerobiosis. J. Rapid Methods Automation Microbiol., 5: 1-12. https://doi.org/10.1111/j.1745-4581.1997.tb00143.x
Adler, H.I., 1990. The use of microbial membranes to achieve anaerobiosis. Crit. rev. Biotechnol., 10: 119-127. https://doi.org/10.3109/07388559009068263
Baron, E.J., Curren, M., Henderson, G., Somer, H.J., Lee, K., Lechowitz, K., Strong, C.A., Summanen, P., Tuner, K. and Finegold, S.M., 1992. Bilophila wadsworthia isolates from clinical specimens. J. clin. Microbiol., 30: 1882-1884.
Bennett, T.P. and Frieden, E., 1969. Modern topics in biochemistry. Macmillan, London, pp. 43-45.
Brodsky, O. and Cronin, C.N., 2006. Economical parallel protein expression screening and scale-up in Escherichia coli. J. Struct. Funct. Genom., 7: 101-108. https://doi.org/10.1007/s10969-006-9013-0
Bryant, M.P., 1972. Commentary on the Hungate technique for culture of anaerobic bacteria. Am. J. clin. Nutr., 25: 1324-1328. https://doi.org/10.1093/ajcn/25.12.1324
Carson, C.A., Shear, B.L., Ellersieck, M.R. and Asfaw, A., 2001. Identification of fecal Escherichia coli from humans and animals by ribotyping. Appl. environ. Microbiol., 67: 1503-1507. https://doi.org/10.1128/AEM.67.4.1503-1507.2001
Cheesbrough, M., 2006. District laboratory practice in tropical countries, Part 2, 2nd Edition. Cambridge University Press, Cambridge, UK. https://doi.org/10.1017/CBO9780511543470
Dao, M.C., Amandine-Everard, J., Wisnewsky, A., Sokolovska, N., Prifti, E., Verger, E.O., Kayser, B.D., Levenez, F., Chilloux, J., Hoyles, L., Dumas, M.E., Rizkalla, S.W., Dore, J., Cani, P.D. and Clement, K., 2016. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut, 65: 426-436. https://doi.org/10.1136/gutjnl-2014-308778
Duncan, S.H., Hold, G.L., Barcenilla, A., Stewart, C.S. and Flint, H.J., 2002. Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. Int. J. Syst. Evol. Microbiol., 52: 1615-1620. https://doi.org/10.1099/00207713-52-5-1615
EMBL, 2018. Protein purification protocol. Web Page accessed at: https://www.embl.de/pepcore/pepcore_services/protein_purification/extraction_clarification/cell_lysates_ecoli/
Feder, I., Wallace, F.M., Gray, J.T., Fratamico, P., Fedorka-Cray, P.J., Pearce, R.A., Call, J.E., Perrine, R. and Luchansky, J.B., 2003. Isolation of Escherichia coli O157:H7 from intact colon fecal samples of swine. Emerg. Infect. Dis., 9: 380-383. https://doi.org/10.3201/eid0903.020350
Feng, Z., Long, W., Hao, B., Ding, D., Ma, X., Zhao, L. and Pang, X., 2017. A human stool-derived Bilophila wadsworthia strain caused systemic inflammation in specific-pathogen-free mice. Gut Pathog., 9: 59. https://doi.org/10.1186/s13099-017-0208-7
Frank, J.A., Reich, C.I., Sharma, S., Weisbaum, J.S., Wilson, B.A. and Olsen, G.J., 2008. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. environ. Microbiol., 74: 2461-2470. https://doi.org/10.1128/AEM.02272-07
Gehring, A.G., George, C.P., Sue, A.R., Shu, I.T. and James, A.L., 2014. Casamino acids and oxyrase enhance growth of Listeria monocytogenes in multi-pathogen enrichments. Fd. Cont., 40: 93-99. https://doi.org/10.1016/j.foodcont.2013.11.038
Hintz, M., Reichenberg, A., Altincicek, B., Bahr, U., Gschwind, R.M., Kollas, A. K., Beck, E., Wiesner, J., Eberl, M. and Jomaa, H., 2001. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett., 509: 317-322. https://doi.org/10.1016/S0014-5793(01)03191-X
Holt, J.G., Krieg, N.R., Sneath, P.H.A., Stanley, J.T. and Williams, S.T., 1994. Bergey’s manual of determinative bacteriology, 9th edition. Lippincott Williams and Wilkins, Philadelphia, PA 19106, USA.
Hungate, R.E., 1969. A roll tube method for cultivation of strict anaerobes. In: Methods in microbiology (eds. J.R. Norris and D.W. Ribbons), Vol. 3B. Academic Press, New York, pp. 117.
James, G.T., 1978. Inactivation of the protease inhibitor phenylmethylsulfonyl fluoride in buffers. Anal. Biochem., 86: 574-579. https://doi.org/10.1016/0003-2697(78)90784-4
Janda, J.M. and Abbott, S.L., 2007. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: Pluses, perils, and pitfalls. J. clin. Microbiol., 45: 2761-2764. https://doi.org/10.1128/JCM.01228-07
Jin, L.Z., Ho, Y.W., Abdullah, N., Kudo, H. and Jalaludin, S., 1997. Studies on the intestinal microflora of chicken under tropical condition. Asian-Australas. J. Anim. Sci., 10: 495-504. https://doi.org/10.5713/ajas.1997.495
Jukes, T.H. and Cantor, C.R., 1969. Evolution of protein molecules. In: Mammalian protein metabolism (ed. H.N. Munro). Academic Press, New York, pp. 21-132. https://doi.org/10.1016/B978-1-4832-3211-9.50009-7
Lineweaver, H. and Burk, D., 1934. The determination of enzyme dissociation constants. J. Am. chem. Soc., 56: 658-666. https://doi.org/10.1021/ja01318a036
Maune, M.W. and Tanner, R.S., 2012. Description of Anaerobaculum hydrogeniformans sp. nov., an anaerobe that produces hydrogen from glucose, and emended description of the genus Anaerobaculum. Int. J. Syst. Evol. Microbiol., 62: 832-838. https://doi.org/10.1099/ijs.0.024349-0
McWilliam, H., Li, W., Uludag, M., Squizzato, S., Park, Y.M., Buso, N., Cowley, A.P. and Lopez, R., 2013. Analysis tool web services from the EMBL-EBI. Nucl. Acids Res., 41: 597-600. https://doi.org/10.1093/nar/gkt376
Ohlendieck, K., 2004. Extraction of membrane proteins. In: Protein purification protocols (ed. P. Cutler). Methods Mol. Biol., 244: 283-293.
Palmer, T. and Bonner, P.L., 2007. Enzymes-biochemistry, biotechnology, clinical chemistry, 2nd edition. Woodhead Publishing, Nottingham Trent University, UK.
Quaranta, M., Borisov, S.M. and Klimant, I., 2012. Indicators for optical oxygen sensors. Bioanal. Rev., 4: 115-157. https://doi.org/10.1007/s12566-012-0032-y
Rattray, F.P. and Fox, P.F., 1997. Purification and characterization of an intracellular esterase from Brevibacterium linens ATCC 9174. Int. Dairy J., 7: 273-278. https://doi.org/10.1016/S0958-6946(97)00013-7
Rehman, A. and Imtiaz, A., 2018. Bacillus subtilus BML5 isolated from soil contaminated with poultry waste has keratinolytic activity. Pakistan J. Zool., 50: 143-148.
Saitou, N. and Nei, M., 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol., 4: 406-425.
Savage, D.F., Anderson, C.L., Colmenares, Y.R., Newby, Z.E. and Stroud, R.M., 2007. Cell-free complements in vivo expression of the E. coli membrane proteome. Prot. Sci., 16: 966-976. https://doi.org/10.1110/ps.062696307
Sawamura, H., Kato, N., Sawa, K., Watanabe, K. and Ueno, K., 1997. Rapid identification of Bilophila wadsworthia. Kansenshogaku Zasshi, 71: 614-619. https://doi.org/10.11150/kansenshogakuzasshi1970.71.614
Schlegel, S., Rujas, E., Ytterberg, A.J., Zubarev, R.A., Luirink, J. and de Gier, J.W., 2013. Optimizing heterologous protein production in the periplasm of E. coli by regulating gene expression levels. Microb. Cell Fact., 12: 24. https://doi.org/10.1186/1475-2859-12-24
Snedecor, G.W. and Cochran, W.G., 1980. Statistical methods, 7th edition. Iowa State University, USA.
Tamura, K., Stecher, G., Peterson, D., Filipski, A. and Kumar, S., 2013. MEGA6: Molecular evolutionary genetics analysis, Version 6.0. Mol. Biol. Evol., 30: 2725-2729. https://doi.org/10.1093/molbev/mst197
Tong, Z., 2011. How to use an avestin emulsiflex C3 homogenizer to disrupt cells. Bio-Protocol, Bio101: e11. https://doi.org/10.21769/BioProtoc.11
Tuitemwong, K., Fung, D.Y.C., and Tuitemwong, P., 1994. Food grade oxygen reducing membrane bound enzymes. J. Rapid Methods Automation. Microbiol., 3: 1-22. https://doi.org/10.1111/j.1745-4581.1994.tb00305.x
Ukkonen, K., Vasala, A., Ojamo, H. and Neubauer, P., 2011. High-yield production of biologically active recombinant protein in shake flask culture by combination of enzyme-based glucose delivery and increased oxygen transfer. Microb. Cell Fact., 10: 107. https://doi.org/10.1186/1475-2859-10-107
Wongjaroen, P., Kittiniyom, K., Srimanote, P., Tiyasuttipan, W. and Wonglumsom, W., 2008. Evaluation of membrane fragments extracted from Escherichia coli and Pseudomonas aeruginosa on Campylobacter jejuni growth under normal atmosphere. Kasetsart J. (Nat. Sci.), 42: 213-218.
To share on other social networks, click on any share button. What are these?










