The Effect of Different Levels of Matricaria chamomilla on Functional Status of the Liver in Rats Injected with Carbon Tetrachloride
The Effect of Different Levels of Matricaria chamomilla on Functional Status of the Liver in Rats Injected with Carbon Tetrachloride
Magbolah Salem Helal Alzahrani
Department of Biology, Faculty of Science, Al-Baha University, Al-Baha, Kingdom of Saudi Arabia
ABSTRACT
Chamomile is a member of the Asteraceae family and has been well established as a medicinal plant. There are many different species of chamomile, the two most common being German chamomile (Marticaria chamomilla) and Roman chamomile (Chamaemelum nobile). The use of chamomile has been associated with calming and anti-inflammatory properties. The plant’s healing properties come from its flowers, which contain volatile oils including bisabolol, bisabolol oxides A and B, and matricin as well as flavonoids. This study aims to elucidate the possible therapeutic applications of different levels of M. chamomilla on the functional status of liver, in rats injected with carbon tetrachloride (CCl4), a known hepatotoxin. Thirty-six male albino rats were treated with subcutaneous injection of CCl4 in paraffin oil 50% V/V (2ml/kg b. wt.) twice a week for 2 weeks to induce chronic liver damage. After injection of CCl4, blood samples were obtained using the retro-orbital method to confirm liver damage and estimate liver function. Rats given CCl4 prior to feeding M. chamomilla demonstrated decrease in serum lipoprotein HDL, LDL, VLDL fraction levels, indicating liver damage. Rats given CCl4 and fed on 5% M. chamomilla showed the most significant increase in organ weight as compared to all levels of treatment suggesting that M. chamomilla can reduce liver damage. Rats given CCl4 then fed on a combination of all levels of M. chamomilla showed a decrease of AST, ALT and ALP enzyme levels in the serum, suggesting a reduction in liver damage. This study showed that proceeding liver damage using CCl4 and administering 5% M. chamomilla, liver function improved significantly as shown with a reduction in serum AST, ALT and ALP, and use of M. chamomilla should be considered in combination therapy for treating liver disease or damage.
Article Information
Received 09 March 2019
Revised 22 June 2019
Accepted 17 August 2019
Available online 25 August 2020
Key words
Matricaria chamomilla, Liver disease, CCl4.
DOI: https://dx.doi.org/10.17582/journal.pjz/20190309080304
* Corresponding author: mshzahrani@bu.edu.sa
0030-9923/2020/0006-2111 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
Introduction
Matricaria chamomilla is a plant species from the Asteraceae family which has medicinal properties. It has been referred to as the star among medicinal species (Singh, 2011). Various functions of the plant has been established which include cosmetic, nutritional and medicinal properties. It has been used for a variety of different ailments including nervousness, anxiety, and headaches. Its anti-inflammatory properties as well as its relaxing and soothing functionalities make it an excellent candidate for use in a variety of medical conditions. Many studies in the literature have researched the use of chamomile to treat a variety of illness’s including small bowel inflammation, intestinal mucositis and rheumatoid arthritis. However, its use in treating liver damage is not as well studied.
Most of the studies pertaining to drug induced toxicity employ liver as a target organ. This is because, liver is responsible for the antioxidative properties of the body as well as metabolism, secretion and storage of substances making it one of the most vital organs of the body (Madrigal-Santillán et al., 2014). Drug and alcohol abuse can cause liver damage and may require transplantation in extreme cases. The use of plant based products has been studied to treat liver disease but little is known about how the use of such products occurs (Guan and He, 2015). CCl4 is well known hepatotoxin and its role of in induction of liver damage and subsequent oxidative stress has been reported elsewhere (Bhattacharjee and Sil, 2007; Aksoy and Sozbilir, 2012).
This investigation aims to study the possible therapy of different levels of M. chamomilla on functional status of liver in rats injected with carbon tetrachloride (CCl4), a well-known liver toxin which has been used widely in research to induce liver damage. Some studies have shown that the use of M. chamomilla can reduce enzymes linked to liver damage (Srivastava et al., 2010). This study examines the serum enzyme levels of CCl4 treated rats, and also to identify the ideal concentration of M. chamomilla in treating liver damage. Using this data a more personalized approach in treating liver damage with M. chamomilla may be applied. This study also examines food intake, bilirubin levels and organ size to ascertain what concentration is ideal in treating liver damage.
Materials and Methods
Plants
M. chamomilla plants were purchased as dried material from the local market, while others were obtained as raw plants from local green groceries.
Basal diet
The basal diet was prepared following standard procedure (Reeves et al., 1993). It consisted of 20% protein (casein), 10% sucrose, 4.7% corn oil, 2% choline chloride, 1% vitamin mixture, 3.5% salt mixture and 5% fiber (cellulose). The remainder was corn starch. The salt mixture comprised (g/100g) CaCO3, 600 mg; K2 HPO4, 645 mg; Ca HPO4. 2H2O, 150 mg; MgSO4.2H2O, 204 mg; NaCl, 334 mg; Fe (C6H5O7) 2.6H2O, 55 mg; Kl, 1.6 mg; MnSO4.4H2O, 10 mg; ZnCl2, 0.5 mg and Cu SO4. 5H2O, 0.06 mg (Hegsted et al., 1941). The vitamin mixture comprised: vitamin E, 10 IU; vitamin K, 0.50 IU; vitamin A, 200 IU; thiamin, 0.50 mg; pyridoxine, 1.00 mg; niacin, 4.00 mg; Calcium panthothenic acid, 0.40 mg; vitamin D, 100 IU; choline chloride, 200 mg; folic acid, 0.02 mg; inositol, 24 mg; para-amino – benzoic acid, 0.02 mg; vitamin B12, 2.00 µg and biotin, 0.02 mg (Campbell,1963).
Experimental diet
The experimental diet consisted of the basal diet plus 10g of different concentrations of M. chamomilla ranging from 5-25%. Each group is outlined below along with concentration added to each basal diet (Table I).
Carbon tetra chloride
Carbon tetrachloride (CCl4) was obtained from El-Gomhoryia Company for Chemical Industries, Cairo, Egypt as 10% liquid solution. It was dispensed in white plastic bottles each containing one liter as a toxic chemical material for liver poisoning according to Passmore and Eastwood, 1986. In the same time, it is mixed with paraffin oil which obtained from the pharmacy for dilution during the induction.
Rats
Mature male albino rats of Sprague-Dawley strain weighing 150-160 g body weight at age of 14-16 weeks were obtained from Laboratory of Animal Colony, Helwan, Egypt. The animals were allocated in plastic cages with metallic stainless covers and kept under strict hygienic measures. Rats were fed with basal diet for 7 days before the beginning of the experiment for adaptation. Diets were presented to rats in special non-scattering feeding cups to avoid loss of food and contamination. Water was provided ad libitum via a narrow mouth bottle with a metallic tube tightly fixed at its mouth by a piece of rubber tube. Animals were subjected to a 12 h light and 12 h dark schedule and kept for 7 days before the start of the experiment for acclimatization as noted before.
Plant preparation
The plant materials were grinded in a mixer to give a powder and were kept in dusky stoppered glass bottles in a cool and dry location to reduce oxidation of their contents.
Grouping and feeding of rats
All the rats were fed for one week on basal diet before starting the experiment, then divided into two main groups, the first group (n= 6 rats) was fed on the basal diet only for 28 days. The rats of second main group (n= 36 rats) were injected subcutaneously by CCl4 to induce liver damage according to Jayasekhar et al. (1997). Rats with liver intoxication were divided into following groups (n= 6 rats): Group 1 is a control positive and fed on basal diet for 28 days, Group 2A received basal diet plus 5% M. chamomilla, Group 2B got basal diet plus 10% M. chamomilla,
Table I.- The composition of basal and experimental diet.
|
Component (g) |
Basal diet |
Concentration of M. chamomilla |
||||
|
5% |
10% |
15% |
20% |
25% |
||
|
Test ingredients |
- |
10 |
10 |
10 |
10 |
10 |
|
Casein |
20 |
20 |
20 |
20 |
20 |
20 |
|
Corn oil |
4.7 |
4.7 |
4.7 |
4.7 |
4.7 |
4.7 |
|
Mineral mix |
3.5 |
3.5 |
3.5 |
3.5 |
3.5 |
3.5 |
|
Vitamin mix |
1 |
1 |
1 |
1 |
1 |
1 |
|
Cellulose |
5 |
5 |
5 |
5 |
5 |
5 |
|
Cholin chloride |
2 |
2 |
2 |
2 |
2 |
2 |
|
Sucrose |
10 |
10 |
10 |
10 |
10 |
10 |
|
Corn starch |
Up to 100 |
Up to 100 |
Up to 100 |
Up to 100 |
Up to 100 |
Up to 100 |
Group 2C fed with basal diet plus 15% M. chamomilla, Group 2D received basal diet plus 20% M. chamomilla and Group 2E fed with basal diet plus 25% M. chamomilla.
Induction of liver intoxication in rats
Thirty-six male albino rats were treated with subcutaneous injection of CCl4 in paraffin oil 50% V/V (2ml / kg B. Wt.) twice a week for 2 weeks to induce chronic damage of the liver according to the method described by Jayasekhar et al. (1997). After the injection of CCl4, blood samples were obtained by retro-orbital method to ensure occurrence of liver injury and to estimate liver function.
Blood sampling
At the end of the experiment period (28 days) rats were sacrificed by ether and anesthesia. Blood samples were obtained by retro-orbital method in a clean dry centrifuge tube. They were left to clot by standing at room temperature for 20 min, and then centrifuged at 1500 r.p.m for 15 min. Serum samples were collected by a dry clean syringe, poured in Wisserman tubes and then kept frozen in a refrigerator at -10ºC for biochemical analysis. Rats were thereafter opened, liver, spleen, heart, lungs and kidneys removed and washed in saline solution, then dried and weighed. Relative weights of mentioned organs were calculated using the following formula:
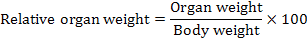
For fixation prior to histopathological investigation, organs were kept in formalin solution (10% V/V) according to methods described by Drury and Wallington (1967).
Biological evaluation
During the period of the experiment, all the rats were weighed once a week and the consumed diets were recorded everyday (daily food intake). At the end of the experiment, biological evaluation of the experimental diets was carried out by determination of body weight gain (%) (BWG%) and food efficiency ratio (FER), according to method of Chapman et al., 1959, using the following formulae:
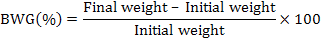

Food intake was also calculated daily.
Determination of the activity of liver enzymes
Determination of aspartate aminotransferase (AST) enzyme was carried out by spectrophotometer using specific kits (BioMerieux) according to Reitman and Frankel (1957). Alkaline phosphatase (ALP) and alanine aminotransferase (ALT) determination was based on colorimetric determination method of Roy (1970). Serum total bilirubin was determined colorimetrically as described by Doumas et al., (1973), using spectrophotometer adjusted at 578 nm.
Determination of components of lipid profile
Total cholesterol was determined according to Ratliff and Hall (1973). Enzymatic colorimetric determination of triglycerides was carried out according to Jacobs and Van Denmark (1960). Determination of HDL was carried out according to the method of Fnedewaid (1972), and Gordon and Amer (1977). The determination of VLDL and LDL was carried out according to the method of Lee and Nieman (1996).
Statistical analysis
The obtained data were statistically analyzed using computerized SPSS (Statistic Program Sigmastat, statistical soft-ware, SAS Institute, Cary, NC). Effects of different treatments were analyzed by one way ANOVA (Analysis of variance) test using Duncan’s multiple range test and p<0.05 was used to indicate significance between different groups (Snedecor and Cochran, 1967).
Results and Discussion
Liver is the vital organ widely employed for toxicity studies. Chemical induced liver damage may be attributed to the toxicity of the parent compound or to the toxic metabolic products (Kuntz and Kuntz, 2005). In the present study CCl4 is utilized for inducing hepatoxicity in rats, owing to its similarity in mechanism of action in humans (Sancheti et al., 2013). Moreover, the hepatoprotectve activity of M. chamomilla against CCl4-induced liver injury in rats have not been reported. The present investigations try to elucidate the hepatoprotective activity of M. chamomilla against CCl4-induced liver injury in rats.
Effect on food intake, body weight gain and feed efficiency ratio
Data presented in Table II shows the effect of different levels of M. chamomilla on food intake (FI), body weight gain % (BWG%) and feed efficiency ratio (FER) in CCl4 - intoxicated rats. It could be observed for rats intoxicated with CCl4 (C+ve) group that FI was significantly reduced (15.05±0.038 g/day) compared to the C-ve normal rats (17.19±0.041 g/day). All rats treated with CCl4 and fed on all tested concentration (5%, 10%, 15%, 20% ,25%) of M. chamomilla had a significant increase in FI. Rats given CCl4 and fed on 10% M. chamomilla showed the most significant increase in FI compared to all levels of M. chamomilla, which reached to 19.09±0.015 g/day.
Table II.- Effects of the different levels of M. chamomilla (5%, 10%, 15%, 20%, 25%) on FI (food intake), BWG% (body weight gain) and FER (feed efficiency ratio) of CCl4-intoxicated rats (n=6rats).
|
Parameters /Groups |
FI (g) |
BWG (%) |
FER |
|
Control – ve |
17.19±0.04d |
35.78±0.97a |
0.13±0.007a |
|
Control + ve |
15.05±0.03g |
10.24±0.62d |
0.06±0.003e |
|
5% M. chamomilla |
17.61±0.02c |
34.04±0.57a |
0.11±0.004b |
|
10% M. chamomilla |
19.09±0.01a |
33.58±0.45a |
0.11±0.006b |
|
15% M. chamomilla |
18.54±0.03b |
24.88±0.64b |
0.09±0.003c |
|
20% M. chamomilla |
16.46±0.02e |
14.37±0.41c |
0.08±0.004c |
|
25% M. chamomilla |
16.19±0.01f |
13.73±0.51c |
0.07±0.004d |
Values denote arithmetic means ± Standard error of the mean. Means with different letters (a, b, c, d) in the same column differ significantly at p≤0.05using one way ANOVA test, while those with similar letters are non-significant.
Concerning body weight gain, the C -ve group was 35.78±0.97% but in C+ve group there was significant reduction at 10.24±0.62%. A significant increase in BWG% in CCl4-intoxicated rats and fed on all levels of M. chamomilla was observed compared to C +ve group. There were non-significant changes between rats fed on 5% and 10% M. chamomilla which were 34.04±0.57 and 33.58±0.45%, respectively. There were also significant changes between rats fed on levels 20% and 25% for BWG% which were 14.37±0.41 and 13.73±0.53%, respectively.
Regarding FER, it was found that in rats injected with CCl4 without treatment (C+ve group), FER was 0.06±0.003 while in normal rats (C-ve) it was 0.13±0.007. These results denote that there was a significant decrease in FER of rats poisoned by CCl4 as compared to normal rats. Rats intoxicated by CCl4 and fed on formulas 5% and10% did not show a significant increase in FER at 0.11±0.004 and 0.11±0.006, respectively. Similarly FER of groups fed with 15% and 20% of M. chamomilla showed values of 0.09±0.006 and 0.08±0.004 respectively, and the differences were non-significant. Meanwhile, rats treated with CCl4 and fed on 25% M. chamomilla showed significant increase in FER compared to C+ve rats which were 0.07±0.004 and 0.06±0.003, respectively.
Dickerson and Lee (1988) reported that many patients with acute or chronic liver disease are ill, and commonly lose weight. Moreover, Clevely and Richmond (1998) concluded that M. chamomilla is medicinally valuable and for treatment of liver disorders.
Effects on relative organs weight
Data listed in Table III shows the effect of feeding at different plant concentration on relative organs weight of CCl4-intoxicated rats. There was a significant decrease in relative liver, spleen, lungs, heart and kidneys weight of rats poisoned by CCl4 as compared to the control (-ve) normal rats. For the relative weight of liver, there were non-significant differences between rats treated with CCl4 and those fed on 10% and 20% concentration of M. chamomilla. CCl4 treated rats fed on 5% concentration of M. chamomilla showed the most significant increase in the relative organ weight as compared to all levels of treatment, while CCl4 treated rats fed on 25% concentration of M. chamomilla showed the lowest significant increase as compared to all levels.
Regarding relative spleen weight, there were non-significant changes between rats injected with CCl4 and fed on 10% and 15% concentration of M. chamomilla. Meanwhile, there were non-significant changes between rats injected with CCl4 and fed on 20% and 25%
Table III.- Effects of feeding with different levels of M. chamomilla on relative organ weight of CCl4–intoxicated rats (n=6rats).
|
Parameters / Groups |
Relative organs weight (g/100 g. B.Wt.) |
||||
|
Liver |
Spleen |
Lungs |
Heart |
Kidneys |
|
|
Control – ve |
3.79±0.08b |
0.62±0.05a |
0.87±0.04a |
0.91±0.04a |
0.94±0.12a |
|
Control + ve |
3.49±0.07e |
0.49±0.05d |
0.67±0.00d |
0.49±0.02d |
0.79±0.07d |
|
5% M. chamomilla |
4.09±0.09a |
0.62±0.01a |
0.83±0.01a |
0.87±0.01a |
0.93±0.06a |
|
10% M. chamomilla |
3.64±0.04c |
0.58±0.01b |
0.75±0.06b |
0.83±0.01a |
0.84±0.08b |
|
15% M. chamomilla |
3.72±0.05b |
0.56±0.03b |
0.74±0.02b |
0.61±0.01c |
0.83±0.02b |
|
20% M. chamomilla |
3.62±0.04c |
0.52±0.01c |
0.72±0.008b |
0.57±0.02c |
0.73±0.007c |
|
25% M. chamomilla |
3.59±0.02d |
0.51±0.01c |
0.69±0.01c |
0.77±0.01b |
0.71±0.009c |
Values denote arithmetic means ± Standard error of the mean. Means with different letters (a, b, c, d) in the same column differ significantly at p ≤ 0.05using one way ANOVA test, while those with similar letters are non-significant.
concentration of M. chamomilla, which were 0.52±0.019 and 0.51±0.011 g/100g B.Wt., respectively. Rats poisoned by CCl4 and fed 5% concentration of M. chamomilla showed the highest significant increase in the mentioned relative organ weight which was 0.62±0.018 g/100g B.Wt., showing non-significant changes as compared to control (–ve) normal rats.
Concerning relative lungs weight, there were non-significant changes between rats injected with CCl4 then fed on levels 10%, 15% and 20% of M. chamomilla. Rats fed on 5% M. chamomilla showed the highest significant increase in lung weight which was 0.83±0.014 g/100g B.Wt. Meanwhile, it did not reflect any significant change as compared to control (-ve) normal rats which was 0.87±0.41 g/100g B.Wt.
Relative heart weight showed no significant changes between rats treated with CCl4 and then fed on 5%, and 10% concentration levels of M. chamomilla. At the same time, there were also non-significant differences between rats given CCl4 then fed on levels 15% and 20%. The highest significant value in mentioned organs were noticed in CCl4 treated rats fed on 5% and 10% M. chamomillaas compared to control (+ve) group.
Kidneys relative weight showed non-significant differences between rats given CCl4 then fed on 10% and 15% concentration of M. chamomilla. Also, there were non-significant changes between rats injured by CCl4 then fed on 20% and 25% of M. chamomilla. CCl4 treated rat s fed on 5% concentration of M. chamomilla showed the highest significant increase among the mentioned relative weights which was 0.93±0.064 g/100g B.Wt., and showed non-significant change as compared to that of (C–ve) normal rats. From the present findings, it can be concluded that carbon tetrachloride CCl4 decreased liver weight and induced the atrophy, however, a diet consisting of 5% and 10% concentration of M. chamomilla can reduced severity of this, and showing an increased organ weight, indicating reduced atrophy from liver damage caused by CCl4. The decrease in liver weight following CCl4 treatment may be attributed to progressive liver damage due to CCl4 induced toxicity (Aksoy and Sozbilir, 2012).
Biochemical analysis
Tables IV, V and VI shows the effect of different concentration levels of M. chamomilla on liver enzymes, total protein and total bilirubin, total cholesterol and triglycerides, and lipoprotein fractions in CCl4-intoxicated rats. Table IV shows the effect of different levels of M. chamomilla on serum liver enzymes including aspartate amino transaminase (AST), alanine amino transferase (ALT) and alkaline phosphatase (ALP) enzymes in CCl4-intoxicated rats. It is reported in the literature that these enzyme markers are used in diagnosis of liver disorders, the concentration of which increases during liver injury or cell damage (Chani and Jasim, 2016). Their increase in the serum has been attributed to the leakage of these enzymes from damaged hepatocytes (Yang et al., 2008).
Table IV.- Effects of different levels of M. chamomilla (5%, 10%,15%,20%,25%) on serum levels of aspartate amino transaminase (AST), alanine amino transferase (ALT) and alkaline phosphatase (ALP) enzymes of CCl4–intoxicated rats (n = 6 rats).
|
Parameters /Groups |
AST (U/L)* |
ALT (U/L)* |
ALP (U/L)* |
|
Control – ve |
65.6±1.8e |
36.5 ± 1.6e |
84.5 ± 1.9e |
|
Control + ve |
130.6 ± 2.1a |
70.5 ± 2.4a |
159.4 ± 2.7a |
|
5% M. chamomilla |
124.6 ± 2.3b |
65.5 ± 2.8b |
154.7 ± 2.5b |
|
10% M. chamomilla |
118.3 ± 2.4c |
46.7 ±2.2c |
146.3 ± 2.8c |
|
15% M. chamomilla |
114.8 ± 2.1c |
40.5 ± 2.6c |
139.5 ± 2.2c |
|
20% M. chamomilla |
113.5 ± 1.6c |
35.5 ± 1.9c |
135.5 ± 1.2c |
|
25% M. chamomilla |
94.7 ± 2.4d |
30.3 ± 2.7d |
114.2 ± 2.9d |
(U/L)*, unit per liter. Values denote arithmetic means ± standard error of the mean. Means with different letters (a, b, c, d) in the same column differ significantly at p ≤ 0.05 using one way ANOVA test, while those with similar letters are non-significant.
It is clear from the Table IV that in rats intoxicated with CCl4 without treatment, the serum levels of AST, ALT and ALP enzymes were 130.6±2.1, 70.5±.4 and 159.4±2.7 U/L, respectively. In (C -ve) normal rats, the serum levels of the mentioned enzymes were 65.6±1.8, 36.5±1.6 and 84.5±1.9 U/L, respectively. In this study there was a significant increase of AST, ALT and ALP enzymes in the serum of rats poisoned by CCl4 as compared to (C –ve) normal rats (Aksoy and Sozbilir, 2012). Rats given CCl4 then fed on different concentration (5%, 10%, 15%, and 20%) of M. chamomilla had a significant decrease in serum levels of AST, ALT and ALP activities. Rats given CCl4 then fed on a combination of all levels of treatment showed a decrease of AST, ALT and ALP enzyme levels in the serum. The decrease in serum AST, ALT and ALP enzyme levels reached to 94.7±2.4, 30.3±2.7 and 114.2±2.9 U/L, respectively. The results corroborate with some of the earlier findings (Troudi et al., 2012; Sancheti et al., 2013; Chain and Jasim, 2016).
In this study, treatment with CCl4 showed a positive result for liver damage while treatment with M. chamomilla at different concentrations rescued this phenotype. An increase in these liver enzymes indicate a pathological phenotype. However, treatment with M. chamomilla
Table V.- Effects of different concentration levels (5%,10%,15%,20%,25%) of M. chamomilla on serum levels of total protein, total bilirubin, total cholesterol and triglycerides of CCl4–intoxicated rats (n = 6 rats).
|
Parameters / Groups |
Total protein (mg/dl) |
Total bilirubin (mg/dl) |
Total cholesterol (mg/dl) |
Triglycerides (mg/dl) |
|
Control – ve |
6.68 ± 1.3a |
0.66 ± 0.01b |
88.98 ± 1.4d |
43.35 ± 1.5d |
|
Control + ve |
4.65 ± 1.6b |
0.99 ± 0.01a |
105.95 ± 1.6a |
56.60 ± 1.9a |
|
5% M. chamomilla |
6.54 ± 1.8a |
0.83 ± 0.01b |
101.97 ± 1.8b |
52.60 ± 1.4b |
|
10% M. chamomilla |
6.55 ± 1.2a |
0.80 ± 0.01b |
98.90 ± 1.2c |
49.50 ± 1.2c |
|
15% M. chamomilla |
6.53 ± 1.3a |
0.75 ± 0.01b |
102.26 ± 1.3b |
54.30 ± 1.4b |
|
20% M. chamomilla |
6.54 ± 1.5a |
0.70 ± 0.01b |
95.90 ± 1.5c |
46.50 ± 1.3c |
|
25% M. chamomilla |
6.53 ± 1.1a |
0.69 ± 0.01b |
90.45 ± 1.1d |
40.50 ± 1.4d |
Values denote arithmetic means ± Standard error of the mean. Means with different letters (a, b, c, d) in the same column different significantly at p ≤ 0.05 using one way ANOVA test, while those with similar letters are non-significant.
at increasing concentrations reduced the levels of these enzymes which are a positive result for treating liver damage and should be considered for patients suffering from this disease. The present results corroborate with the findings of Aksoy and Sozbilir (2012). Whereby, ALT and AST enzymes were significantly increased in the CCl4 group indicating liver damage, but simultaneous treatment with M. chamomilla reduced oxidative stress and antioxidant activity.
Effects on total protein and total bilirubin
Table V shows the effects of different concentration levels of M. chamomilla on serum levels of total protein, total bilirubin, total cholesterol and triglycerides of CCl4- intoxicated rats. Serum levels of total protein and total bilirubin in C +ve group were 4.65±1.6 and 0.99±0.012 mg/dl, respectively. While in the C –ve normal rats were 6.68±1.3 and 0.66±0.011 mg/dl, respectively. These results revealed a significant decrease in total protein but significant increase in total bilirubin in the serum of rats intoxicated by CCl4 as compared to C –ve normal rats. The result is in consistent with earlier findings, demonstrating increased serum total bilirubin and decreased serum total protein in CCl4-induced hepatotxicity in rats (Sancheti et al., 2013). In CCl4 treated rats fed on all levels of M. chamomilla, there was a significant increase in the serum level of total protein, but a significant decrease in serum level of total bilirubin. However, between groups there was a not significant change, which suggests that concentrations of M. chamomilla does not affect the total protein and bilirubin levels and is not effective for this purpose. As increased bilirubin levels are an indicator of liver damage it may be necessary to use higher concentrations of M. chamomilla to achieve a significant decrease in bilirubin levels, so as to aid in the treatment of liver damage. Thus, enhancements in serum ALT, AST and total bilirubin is an indicative of CCl4-induced hepatic injury in rats (Drotman and Lawhorn, 1978). M. chamomilla caused significant decreased the levels of serum ALT, AST and total bilirubin, suggesting its protective function against CCl4 –induced liver damage.
In rats injected with CCl4 and without treatment (C +ve), levels of total cholesterol and triglycerides were 105.95±1.6 and 56.60±1.9 mg/dl, compared to 88.98±1.4 and 53.35±1.5 mg/dl in normal rats (C –ve). The obtained results showed that there was a significant increase in serum levels of total cholesterol and triglycerides in rats poisoned by CCl4 as compared to normal rats. In CCl4 treated rats fed on all levels of treatment, there was a significant decrease in serum levels of total cholesterol and triglycerides as compared to C +ve group. The result corroborate with earlier findings (Nargesi et al., 2018). The lowest levels of cholesterol and triglycerides were observed in the group treated with 25% of M. chamomilla.
There were non-significant differences between 5% and 15% M. chamomilla which were 101.97±1.8 and 102.26±1.3 mg/dl for total cholesterol and were 52.60±1.4 and 54.30±1.4 mg/dl, respectively for triglycerides. Also, there were non-significant differences between 10% and 20% M. chamomilla which showed 98.90±1.2 and 95.90±1.5 mg/dl for total cholesterol and 49.50±1.2 and 46.50±1.3 mg/dl, respectively for triglycerides. Meanwhile, 25% M. chamomilla showed the highest significant decrease both in total cholesterol and triglycerides as compared to all levels of treatment, and revealed non-significant differences compared with (C –ve) normal rats as regards both parameters. Similar results were observed by Tsi and Tan (1996).
Table VI shows the effects of different concentration levels of M. chamomilla on the serum levels of lipoprotein fraction (HDLc, LDLc and VLDLc) in CCl4–intoxicated rats. There was a significant increase in HDLc, LDLc and VLDLc lipoprotein fractions in the serum of rats poisoned by CCl4 and without treatment as compared to the control (–ve) normal rats. In C +ve rat serum levels of HDLc, LDLc and VLDLc were 75.69±1.2, 18.64±1.4 and 11.32±1.6 mg/dl, respectively. In normal rats (C –ve) the serum levels of lipoprotein fractions were 63.96±1.1, 16.35±1.2 and 8.67±1.1 mg/dl, respectively. Rats injected with CCl4 and fed on 10% and 20% M. chamomilla had a significant decrease in high density lipoprotein (HDL-c) while those fed on all levels had a significant decrease in low and very low density lipoprotein (LDLc and VLDLc) as compared to control +ve group. The result is consistent with previous studies (Nargesi et al., 2018). With regard to Lipoprotein fraction HDL, there was no significant differences between C +ve rats treated with CCl4 and fed on 5% and 15% concentration of M. chamomilla compared to control (+ve) group which were 74.75±1.3, 73.70±1.7 and 75.69±1.2 mg/dl, respectively. Also, rats injured by CCl4 and fed on 5%, 10% and 20% M. chamomilla showed lipoprotein fraction LDLc of 16.70±1.3, 16.90±1.3 and 16.50±1.4 mg/dl, respectively. Regarding lipoprotein fraction VLDc, there were non-significant differences between rats given CCl4 then fed on 5% and 15%M. chamomilla, which were 10.52±1.8 and 10.86±1.8 mg/dl, respectively. Also, there were non-significant differences between rats given CCl4 and fed on 10% and 20% M. chamomilla, which were 9.90±.1 and 9.30±1.1 mg/dl, respectively. Finally, rats treated with CCl4 prior to feeding on all levels of treatments revealed the highest decrease in serum lipoprotein HDL, LDL, VLDL fraction levels which reached to 68.85±1.6, 13.50±1.3 and 8.10±1.2 mg/dl, respectively. Similar results were obtained by
Table VI.- Effects of different concentration levels (5%,10%,15%,20%,25%) of M. chamomilla on the serum levels of lipoprotein fractions (HDLc, LDLc and VLDLc) in CCl4-intoxicated rats (n=6 rats).
|
Parameters / Groups |
Lipoprotein fractions (mg/dl) |
||
|
HDLc |
LDLc |
VLDLc |
|
|
Control – ve |
63.96 ± 1.1e |
16.35 ± 1.2c |
8.67 ± 1.1d |
|
Control + ve |
75.99 ± 1.2a |
18.64 ± 1.4a |
11.32 ± 1.6a |
|
5% M. chamomilla |
74.75 ± 1.3b |
16.70 ± 1.3c |
10.52 ±1.8b |
|
10% M. chamomilla |
72.10 ± 1.3c |
16.90 ± 1.3c |
9.90 ±1.1c |
|
15% M. chamomilla |
73.70 ± 1.7b |
17.70 ± 1.3b |
10.86 ± 1.8b |
|
20% M. chamomilla |
70.10 ± 1.2c |
16.50 ± 1.4c |
9.30 ± 1.1c |
|
25% M. chamomilla |
68.85 ± 1.6d |
13.50 ± 1.3d |
8.10 ±1.2d |
Values denote arithmetic means ± Standard error of the mean. Means with different letters (a, b, c, d) in the same column different significantly at p ≤ 0.05using one way ANOVA test, while those with similar letters are non-significant.
Williams et al, 2014. It has been reported elsewhere that M. chamomilla reduces intestinal absorption of lipids (Tavakol et al., 2014).
Thus M. chamomilla seem to be potent hepatoprotective agent in maintaining healthy liver, elucidating its possible therapeutic applications. However, further in-depth studies on histological aspects are warranted to confirm their pharmacotherapeutic significance.
Conclusion
This study showed that different concentration levels of M. chamomilla in the diet of rats suffering from liver damage aided in their recovery and decreased pathological levels of enzymes and cholesterol in the body. However, the use of M. chamomilla to decrease bilirubin levels was not significant, indicating that a higher concentration of M. chamomilla in the diet may be required in some cases. The only limitation in the present study is the histological examination of liver sections of M. chamomilla administered mice. Further studies on histological properties may expose positive results on degenerative changes of liver tissues. Overall findings indicate that appropriate tuning of doses of M. chamomilla is required for different treatment purposes. This study proves that plant materials possess the ability to aid in improving liver function primarily by reducing serum liver enzymes. The use of M. chamomilla would be beneficial for patients with liver disorders but 5-10% concentration would be the most effective in this case.
Statement of conflict of interest
The authors declare no conflict of interest.
References
Aksoy, L. and Sozbilir, N.B., 2012. Effects of Matricaria chamomilla L. on lipid peroxidation, antioxidant enzyme systems, and key liver enzymes in CCl4-treated rats. Toxicol. environ. Chem., 94: 1780-1788. https://doi.org/10.1080/02772248.2012.729837
Bhattacharjee, R. and Sil, P.C., 2007. Protein isolate from the herb, Phyllanthus niruri L. (Euphorbiaceae), plays hepatoprotective role against carbon tetrachloride induced liver damage via its antioxidant properties. Fd. Chem. Toxicol., 45: 817–826. https://doi.org/10.1016/j.fct.2006.10.029
Chapman, D.G., Castilla, R. and Campbell, J.A., 1959. Evaluation of protein in food. I: A method for the determination of protein efficiency ratio. Can. J. Biochem. Phosiol., 37: 679-686. https://doi.org/10.1139/y59-074
Campbell, J.A., 1963. Methodology of protein evaluation. RAG Nutr., Document R.10, Led. 37., New York.
Chani, J.M. and Jasim, N.Z., 2016. Hepatoprotective effect of Matricaria chamomilla hot aqueous extract against methomyl 90%-induced hepatotoxicity in mice. Al-Kufa Univ. J. Biol., 8: 185-195.
Clevely, A. and Richmond, K., 1998. The new guide to herbs. Lorenz Books, Anness Publishing Limited.
Dickerson, J.W. and Lee, H.A., 1988. Nutrition in the clinical management of disease, Second edition. Edward Arnold.
Doumas, B.T., Ferry, B.W., Sasse, E.A. and Straum, J.V., 1973. Cited in the pamphlet of Quimica. Clin. Apli. Amp. Spain. Clin. Chem., 19; 984-993.
Drotman, R.B. and Lawhorn, G.T., 1978. Serum enzymes as indicators of chemical induced liver damage. Drug Chem. Toxicol., 1: 163–171. https://doi.org/10.3109/01480547809034433
Drury, R.A. and Wallington, E.A., 1967. Carton’s histological technique, 5th ed. Oxford University Press.
Fnedewaid, W.T., 1972. Determination of HDL. Clin. Chem., 18: 499.
Gordon, T. and Amer, M., 1977. Determination of HDL. J. Med., 62: 707.
Guan, Y.S. and He, Q., 2015. Plants consumption and liver health. Evidence-Based Complem. Altern. Med., 2015: Article ID 824185. https://doi.org/10.1155/2015/824185
Hegsted, D., Mills, R. and Perkins, E., 1941. Salt mixture. J. biol. Chem., 138: 459.
Jacobs, N.J. and van Denmark, P.J., 1960. Determination of triglycerides. Arch. Biochem. Biophys., 88: 250-255. https://doi.org/10.1016/0003-9861(60)90230-7
Jayasekhar, P., Mohanan, P.V. and Rahinam, K., 1997. Hepatoprotective activity of ethyl acetate extract of Acacia catechu. Indian J. Pharmacol., 29: 426-428.
Kuntz, E. and Kuntz, H.D., 2005. Hepatology: History, morphology, biochemistry, diagnostics, clinic, therapy. Birkhäuser, Heidelberg, Germany, pp. 52–53.
Lee, R.D. and Nieman, D.C., 1996. Nutritional assessment, 2nd ed. Mosby, Missoun, USA.
Passmore, R. and Eastwood, M.A., 1986. Human nutrition and dietetics, Eight edition. Longman Group Ltd., Churchill Livingstone, UK.
Madrigal-Santillán, E., Madrigal-Bujaidar, E., Álvarez-González, I., Sumaya-Martinez, M.T., Gutierrez-Salinas, J., Bautista, M., Morales-Gonzalez, A., Garcia-Luna, M., Gonzalez-Rubio, Y., Aguilar-Faisal, J.L. and Morales-Gonzalez, J.A., 2014. Review of natural products with hepatoprotective effects. World J. Gastroenterol., 20: 14787-14804.
Nargesi, S., Moayeri, A., Ghorbani, A., Seifinejad, Y., Shirzadpour, E. and Amraei, M., 2018. The effects of Matricaria chamomilla L. hydroalcoholic extract on atherosclerotic plaques, antioxidant activity, lipid profile and inflammatory indicators in rats. Biomed. Res. Ther., 5: 2752-2761. https://doi.org/10.15419/bmrat.v5i10.490
Ratliff, C.R. and Hall, F., 1973. A new method for direct colorimetric determination of serum cholesterol. In: Laboratory manual of clinical biochemistry. Scoot and White Memorial Hospital Publications, Temple, TX, USA.
Reeves, P.G., Nielson, F.H. and Fahmy, G.C.,1993. Reports of the American Institute of Nutrition, adhoc wiling committee on reformulation of the AIN 93. Rodent Diet. J. Nutri., 123: 1939-1951. https://doi.org/10.1093/jn/123.11.1939
Reitman, S. and Frankel, S., 1957. Colorimetric method for aspartate and alanine aminotransferase. Am. J. clin. Path., 28: 26. https://doi.org/10.1093/ajcp/28.1.56
Roy, S.E., 1970. Colorimetric determination of serum alkaline phosphatase. Clin. Chem., 16: 431-432.
Sancheti, S., Sancheti, S. and Seo, S.Y., 2013. Ameliorative effects of 7-methylcoumarin and 7-methoxycoumarin against CCl4-induced hepatoxicity in rats. Drug Chem. Toxicol., 36: 42-47. https://doi.org/10.3109/01480545.2011.648329
Snedecor, G.W. and Cochran, W.G., 1967. Statistical methods, 6th ed. Iowa State University Press, Ames, Iowa, USA.
Singh, O., Khanam, Z., Misra, N. and Srivastava, M.K., 2011. Chamomile (Matricaria chamomilla L.): An overview. Pharmacog. Rev., 5: 82-95. https://doi.org/10.4103/0973-7847.79103
Srivastava, J.K., Shankar, E. and Gupta, S., 2010. Chamomile: A herbal medicine of the past with bright future. Mol. Med. Rep., 3: 895-901. https://doi.org/10.3892/mmr.2010.377
Tavakol, H.S., Farzad, K., Fariba, M., Abdolkarim, C., Hassan, G., Seyed-Mostafa, H.Z. and Akram, R., 2014. Hepatoprotective effect of Matricaria chamomilla L. in paraquat induced rat liver injury. Drug Res., 64: 1-5. https://doi.org/10.1055/s-0033-1363999
Troudi, A., Amara, I., Samet, A. and Zeghal, N., 2012 Oxidative stress induced by 2, 4-phenoxyacetic acid in liver of female rats and their progeny: Biochemical and histopathological studies. Environ. Toxicol., 27: 137-145. https://doi.org/10.1002/tox.20624
Tsi, D. and Tan, B.K., 1996. Effects of celery extract and 3-N-butylphthalide on lipid levels in genetically hypercholesterolaemic (RICO) rats. Clin. exp. Pharmacol. Physiol., 23: 214-217. https://doi.org/10.1111/j.1440-1681.1996.tb02598.x
Williams, J.A., Manley, S. and Ding, W.X., 2014. New advances in molecular mechanisms and emerging therapeutic targets in alcoholic liver diseases. World J. Gastroenterol., 20: 12908-12933. https://doi.org/10.3748/wjg.v20.i36.12908
Yang, Y.S., Ahn, T.H, Lee, J.C., Moon, C.J., Kim, S.H., Jun, W., Park, S.C., Kim, H.C. and Kim, J.C., 2008. Protective effects of Pycnogenol on carbon tetrachloride-induced hepatotoxicity in Sprague Dawley rats. Fd. Chem. Toxicol., 46: 380–387. https://doi.org/10.1016/j.fct.2007.08.016
To share on other social networks, click on any share button. What are these?










