Rodents (Muridae) Abundance and Habitat Shift between Agricultural, Non-Agricultural Land and Human Dwellings: A Proposed Strategy to Decrease Crop Damage
Rodents (Muridae) Abundance and Habitat Shift between Agricultural, Non-Agricultural Land and Human Dwellings: A Proposed Strategy to Decrease Crop Damage
Raja Imran Hussain* and Iqra Yousaf
Department of Biology, PMAS-Arid Agriculture University, Rawalpindi
ABSTRACT
Rodents (Muridae) are an important pest and their uncontrolled dispersal threatening yields of various crops including wheat. The goal of this study was to evaluate the shifts of rodent abundances among five sets of three different habitat types (human dwellings, agricultural and non-agricultural land). It was also expected that trapping in non-agricultural land and human dwellings could reduce wheat damage. 30 trap stations were placed in each cropland, non-agricultural land and human dwellings for twelve months. Rodent abundance was high in cropland and negatively correlated with human dwellings. No difference in abundance was observed in the non-crop and fallow land. Tatera indica was the most abundant species. High adjusted trapping success in cropland and low in human dwellings were observed during the spring season. Trapping influenced by 4.63% decrease in wheat damage. Non-agricultural land and human dwellings provide good shelter and food resources for rodent dispersal. Controlling one habitat of the landscape, either human dwellings or cropland, might not allow effective results because rodents still have alternative habitat for survival. Therefore, it is recommended to apply the control strategy on both habitat types before sowing the wheat crop. This might prevent damage to wheat and possible reduction of intensive use of rodenticides in cropland.
Article Information
Received 12 October 2018
Revised 15 November 2018
Accepted 22 November 2018
Available online 07 August 2019
Authors’ Contributions
RIH and IY did the sampling. IY statistically analyzed the data. RIH wrote the manuscript.
Key words
Rodent control, Habitat use, Seasonal abundance, Crop damage, Yield loss.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.6.2051.2056
* Corresponding author: rajaimran2003@gmail.com
0030-9923/2019/0006-2051 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Among mammals, rodents are the most diverse group (Nowak, 1999; Singleton et al., 2003), representing almost 40% of all mammals on the Earth (Nowak, 1999). Two third of living rodents species belong to just family, Muridae, which is the largest in the whole class of mammalian and most of them found in Asia (Aplin et al., 2003). Eighteen species of rodents (Muridae) are considered pest in the agriculture, human dwellings and rural and urban storage facilities (Parshad, 1999). They significantly damage vast range of agricultural crops such as wheat (damaged by Bandicota spp.; Poche et al., 1982), rice (by Mastomys natalensis; Islam et al., 1993; Singleton et al., 2005) and maize (by Xerus erythropus; Mulungu et al., 2003). They also caused damage at all stages of wheat crop by digging up newly planted seeds, cutting tillers to gain access to nutrients or accessing the developing grain as the crop matures (Brown et al., 2007). The vast wheat damage is due to the high rate of reproduction, adaptability and poor control strategies (Parshad, 1999).
Usually, control measures are initiated after damage by rodents is recognized. However, few studies have actually demonstrated the assumption of the damage is reduced after animals were killed (Advani and Mathur, 1982; Sheikher and Jain, 1997). The distribution and pattern of relative abundance of rodents depend largely on the seasons and availability of food and water sources (Brown and Ernest, 2002). Cereal crops provide food for many rodents during the whole growing season (Addisu and Bekele, 2013) and human dwelling expected to provide shelter to them (Kotler, 1984).
Seasonality (Datiko et al., 2007) and habitat selection of rodents are considered to be important factors shaping the community dynamics and dispersal of rodents (Oguge, 1995; Churchfield et al., 1997). Our study aimed to bring human dwellings, agricultural and non-agricultural land together in one study to understand how rodents abundance shifted among them. Seasonality also predicted to play a critical role in rodents abundance shift. Also, we hypothesise that trapping, throughout the whole year will reduce wheat damage.
Materials and methods
Study area
Sampling sites were located in the rural area of Islamabad city, which is used for wheat production (Khan et al., 2009; Hussain et al., 2017). The land consists of scattered trees of Ziziphus jujuba, Dalbergia sisso, Melia azadirachta and Acacia nilotica and other wild plants species included Carissa opaca, Calotropis procera, Adhatoda zeylanica, Imperata cylindrica, Cannabis sativa and Cynodon dactylon. According to soil analysis, soil types were slightly alkaline (pH 7.3-8.73).
Climate data
Temperature and rainfall were considered as a limiting factor for rodents (Dickmana et al., 1999). Data about average annual rainfall and temperature of each month is collected by the regional meteorological center (Supplementary Table I).
Sampling method
Rodent abundance was assessed using the trap-line methods which are considered as a variant of the line transect method (Rudran et al., 1996). The study area included five sets of cropland (annually sowing wheat crop), non-crop (nearby land which are not defined as crop land) and fallow land (field left for one year to attain its fertility and used again for sowing wheat crop). When refereeing to the field then it includes crop, non-crop and fallow land together. 30 snap trap stations were placed in a straight line per habitat type. The selected fields were equal in size and adjacent to each other (Fig. 1). Seven houses were selected about 400~500 m distance parallel to the sampling fields. Three snap traps (two large rat trap and one small mouse trap) were set up together with fresh potatoes and chapatti at each station. Each snap trap was tied with a rope (2 m length) and connected with a single hook that was inserted into the soil, in the center of three snap traps. The distance between each trap station was 30 m. Trapping was done in three nights, in the middle of each month, throughout the whole year (May 2014-April 2015). Transects starting point were chosen randomly on each trapping section. Trapping around human dwellings was conducted coincidently with samplings in the cropland and non-crop land.
A total of 8 plots at 10m of distance (each 90 m2) were selected within cropland and non-agricultural land. In each plot, 25 tiller samples were collected diagonally using a wooden frame. In each sample, we registered the sum of damaged and undamaged tillers inside the frame. Samplings were done before wheat harvesting in 2014 and 2015. According to Poche et al. (1982), the damage percentage was calculated as follow:
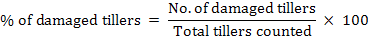
When assessing crop damage, the same person surveys all the data to avoid assessment bias. Trap stations were set at dusk and visited the next morning before the sunrise to get population abundance and to avoid any harm to birds that might be attracted by bait. Specimen were collected from traps and frozen for identification (Roberts, 1997).
Data analysis
Percentage trap success was calculated as the ratio between the number of rodents captured with the total number of traps set, multiplied by 100 (Chitty and Shorten, 1946). There was one trap less accessible whenever an animal was caught in a trap, so the number of active traps reduced on every sampling night. This was adjusted by calculating adjusted trap success (Caughley, 1977). Rodent density index (RDI) was calculated following Busch et al. (2011) method:
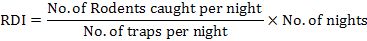
Generalized linear model (GLM) with Poisson error distribution was used to find the difference between the response variable (number of individuals) and predictor variable (season, habitat). The predictor variables are 2 fixed factors: Season (with four levels) and habitat type (with 3 levels). The data were checked for normality, homogeneity and over dispersion, and if found then corrected by using quasi-GLM (Hussain et al., 2018; Rebbah et al., 2019). Pearson correlations were computed to determine covariant in the data structure (Hussain et al., 2018). We analyzed sites data for autocorrelation (Walcher et al., 2017). We analyzed rodents data captured in dwellings separately from those captured in fields. All statistical tests were performed in R version 3.31 (R Core Team, 2016) by using an alpha level of 0.05.
Results
A total of 429 rodents were collected from 3240 effective trap-nights. 193 rodents were captured in human dwellings and 236 from fields. Overall rodent numbers fluctuate per month (Fig. 2). The higher in April (32) and the lowest in December (11) was found in fields while reverse trend were observed in human dwellings. In total, Tatera indica (73%), Mus musculus (20%) and Bandicota bengalensi (7%) species were the most abundant species, which were reported consistently in sampling region (Mehmood et al., 2011). A significant difference was observed between the number of individuals and spring seasons in the fields (GLM: Residual deviance: 12.439, df=8, P=0.001; Fig. 3A) and in the human dwellings (GLM: Residual deviance: 5.23, df=8, P=0.005; Fig. 3B). There was significantly higher number of individuals in cropland than in human dwellings (GLM: Residual deviance: 19.716, df=10, P=0.003). Spring and summer seasons showed inverse variability in cropland and non-crop land. Higher ATS was observed in fields and low in human dwellings during spring season. The RDI of rodents ranged from 0.10 to 0.30 in both fields and in human dwellings (Fig. 4). This was similar between summer and winter seasons, while in spring, it differed between fields (0.30) and human dwellings (0.10). Reduction of wheat damage was observed from 26.98% to 22.35% in the cropland (Supplementary Table II). We did not find any autocorrelation between sites (p >0.05).
Discussion
Rodents caused damage to different stages of the wheat plants either by cutting tillers of young plants to gain access to the nutritional content present in the tiller or to obtain a fully mature grain (Brown et al., 2007). High rodent abundance was seen from December to April (from newly wheat plant to developed grain). The sudden increase of rodent abundance from December to April in the crop field can be linked with the roots of wheat plants that started to grow up, sweeten and ended with grain. This may attract more rodents towards cropland and cause an increase in abundance. In contrast, a decline in abundance was found in the human dwelling from December to April. Farmers moved the major quantity of grains to the fields for sowing which reduced food availability for rodents
in human dwellings. A strong negative correlation was observed between rodents abundance in cropland and in human dwellings (r = -0.73). It seems that rodents follow the path of food sources and dispersed accordingly.
The overall trap and adjusted trap success varied with seasons. The lowest success was obtained from autumn in fields and from spring in the human dwellings. This may be linked to annual temperature and rainfall. In summer, rodents abundance increased in the non-crop field and human dwellings. The high temperature was observed in the summer season (37oC) that made crop land more difficult for rodents survival. Later, Monsoon heavy rain water stayed in the cropland that might cause rodents shifted.
Rodents need food, shelter (Witmer et al., 2007) or a buffer zone (Yletyinen and Norrdahl, 2008) either provided by the nearby non-agricultural land or human dwellings. Rodents abundance started increasing when a wheat plant was large enough to cover field soil. This high ground vegetation cover might be a suitable place for shelter from predators and reproduction. Rodent absence after harvesting crop fields did not mean that their population is controlled. Observing data from human dwellings and non-agricultural land, it might be possible that rodents prefer to disperse towards more secure areas in term of availability of food and shelter.
Wheat damage was reduced from 4.63% after two harvesting sessions. Continuous sampling per month might reduce this and it is expected that continuous management of rodents in human dwellings and non-agricultural land will further decrease such damage. Overall it is suggested to apply rodent control strategy in nearby non-agricultural land and human dwellings before sowing wheat crop to control rodent dispersal and wheat damage. It is worthful to mention the need for farmers to secure their seed grain. As rodents left structures when the grain was planted, it is reasonable to suggest that a real key would be securing seed pre-sowing to prevent maintenance of a rodent population in areas with human dwellings.
Conclusion
Rodent abundance varies with the food resources and seasons. Our results indicate that fewer rodents in cropland did not mean that it is protected, instead, rodents hibernate in nearby non-agricultural land and human dwellings having adequate food sources and shelter. For rodents control strategy, it will be a good idea to securing seed pre-sowing, control surrounding well shaded non-agricultural land and human dwellings that could be a possible place for rodents to hide and reproduce. This might prevent severe damage and reduce the indiscriminate use of rodenticides to control the damage that already occurred. Rodent control of just one area, either human dwellings or cropland, might not give effective results because rodents still have alternative habitat for survival. This strategy possibly reduces wheat damage by rodents and increase wheat production. Further, attempts should be made to estimate the size of rodent infestation and damage in the long-term scale.
Acknowledgements
We would like to thanks Raja Muhammad Noman and Jhangir Alam for providing help during trapping. We are grateful to Marcela Suarez-Rubio for assistance and fruitful discussions. Special thanks to the farmers and house owners for their permission to conduct investigations on their fields and houses. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
There is supplementary material associated with this article. Access the material online at: http://dx.doi.org/10.17582/journal.pjz/2019.51.6.2051.2056
Statement of conflict of interest
The authors declare no conflict of interest.
References
Addisu, A. and Bekele, A., 2013. Habitat preferences, seasonal abundance and diets of rodents in Alage, Southern Ethiopia. Afr. J. Ecol., 52: 284-291.
Advani, R. and Mathur, R.P., 1982. Experimental reduction of rodent damage to vegetable crops in Indian villages. Agro-Ecosyst., 8: 39-45. https://doi.org/10.1016/0304-3746(82)90013-0
Aplin, K., Brown, P., Jacob, J., Krebs, C. and Singleton, G., 2003. Field methods for rodent studies in Asia and the Indo-Pacific. Australian Centre for International Agricultural Research, Canberra, Australia.
Brown, J.H. and Ernest, S.K.M., 2002. Rain and rodents: Complex dynamics of desert consumers. AIBS Bull., 52: 979-987.
Brown, P.R., Huth, N.I., Banks, P.B. and Singleton, G.R., 2007. Relationship between abundance of rodents and damage to agricultural crops. Agric. Ecosyst. Environ., 120: 405-415. https://doi.org/10.1016/j.agee.2006.10.016
Busch, M., Knight, C., Mazía, C.N., Hodara, K., Muschetto, E. and Chaneton, E., 2011. Rodent seed predation on tree invader species in grassland habitats of the inland Pampa. Ecol. Res., 27: 369-376. https://doi.org/10.1007/s11284-011-0909-1
Caughley, G., 1977. Analysis of vertebrate populations. John Wiley and Sons, New York, pp. 234.
Chitty, D. and Shorten, M., 1946. Technique for the study of the Norway rat (Rattus norvegicus). J. Mammal., 27: 63-78. https://doi.org/10.2307/1375143
Churchfield, S., Hollier, J. and Brown, V.K., 1997. Community structure and habitat use of small mammals in grasslands of different successional age. J. Zool., 242: 519-530. https://doi.org/10.1111/j.1469-7998.1997.tb03853.x
Datiko, D., Bekele, A. and Belay, G., 2007. Species composition, distribution and habitat association of rodents from Arbaminch forest and farmlands, Ethiopia. Afr. J. Ecol., 45: 651-657. https://doi.org/10.1111/j.1365-2028.2007.00789.x
Dickmana, C.R., Mahona, P.S., Mastersab, P. and Gibsonb, D.F., 1999. Long-term dynamics of rodent populations in arid Australia: the influence of rainfall. Wildl. Res., 26: 389-403. https://doi.org/10.1071/WR97057
Hussain, R.I., Yousaf, I. and Jernej, I., 2017. Frequency and recommendation to control dog bite injuries in Islamabad Pakistan. Gomal Univ. J. Res., 33: 78-84. https://doi.org/10.14411/eje.2018.014
Hussain, R.I., Walcher, R., Brandl, D., Jernej, I., Arnberger, A., Zaller, J. G. and Frank, T., 2018. Influence of abandonment on syrphid assemblages in mountainous meadows. J. appl. Ent., 142: 450-456. https://doi.org/10.1111/jen.12482
Hussain, R.I., Walcher, R., Brandl, D., Arnberger, A., Zaller, J.G. and Frank, T., 2018. Efficiency of two methods of sampling used to assess the abundance and species diversity of adult Syrphidae (Diptera) in mountainous meadows in the Austrian and Swiss Alps. Eur. J. Ent., 115: 150-156. https://doi.org/10.14411/eje.2018.014
Islam, Z., Morton, R.G. and Jupp, B.P., 1993. The effects of rat damage on deepwater rice yields in Bangladesh. Int. J. Pest Manage., 39: 250-254. https://doi.org/10.1080/09670879309371799
Khan, S.B., Ahmad, F., Sadaf, S. and Kashif, R.H., 2009. Crops area and production (by districts) (1981-82 To 2008-09) Volume I food and cash crops. Federal Bureau of Statistics (Economic Wing), Statistics Division, Government of Pakistan, Islamabad.
Kotler, B.P., 1984. Risk of predation and the structure of desert rodent communities. Ecology, 65: 689-701. https://doi.org/10.2307/1938041
Mehmood, A., Ansari, M.S., Hussain, T., Akhter, S., Khan, S.A., Hassan, S., Khan, A.A. and Rakha, B.A., 2011. Bandicoot Rat (Ban dicota bengalen sis): A Novel Reservoir of Pathogenic Bacteria at Poultry Farms, Rawalpindi/Islamabad, Pakistan. Pakistan J. Zool., 43: 201-202.
Mulungu, L.S., Makundi, R.H., Leirs, H., Massawe, A.W., Vibe-Petersen, S. and Stenseth, N.C., 2003. The rodent density-damage function in maize fields at an early growth stage. ACIAR Monogr. Ser., 96: 301-303.
Nowak, R.M., 1999. Walker’s mammals of the world. Johns Hopkins University Press, Maryland, United States.
Oguge, N.O., 1995. Diet, seasonal abundance and microhabitats of Praomys (Mastomys) natalensis (Rodentia: Muridae) and other small rodents in a Kenyan sub-humid grassland community. Afr. J. Ecol., 33: 211-223. https://doi.org/10.1111/j.1365-2028.1995.tb00798.x
Parshad, V.R., 1999. Rodent control in India. Integr. Pest Manage. Rev., 4: 97-126. https://doi.org/10.1023/A:1009622109901
Poche, R.M., Mian, Y., Haque, E. and Sultana, P., 1982. Rodent damage and burrowing characteristics in Bangladesh wheat fields. J. Wildl. Manage., 46: 139-147. https://doi.org/10.2307/3808416
R Core Team, 2016. R: A language and environment for statistical computing, Version 3.1.1. Retrieved from: http://www.R-project.org
Rebbah, A.C., Menaa, M., Telailia, S., Saheb, M. and Maazi, M.C., 2019. Effect of habitat types on breeding bird assemblages in the Sidi Reghis Forests (Oum El Bouaghi, North-Eastern Algeria). Pakistan J. Zool., 51: 399-799. http://dx.doi.org/10.17582/journal.pjz/2019.51.2.433.447
Roberts, T.J., 1997. Mammals of the Pakistan. Oxford University Press, Karachi, Pakistan.
Rudran, R., Kunz, T.H., Southwell, C., Jarman, P. and Smith, A.P., 1996. Observational techniques for nonviolent mammals. Smithsonian Institution Press, Washington, DC, pp. 81-104.
Sheikher, C. and Jain, S.D., 1997. Rodents in cauliflower and cabbage: Population, damage and control. Int. J. Pest. Manage., 43: 63-69. https://doi.org/10.1080/096708797229004
Singleton, G.R., Hinds, L.A., Krebs, C.J. and Spratt, D.M., 2003. Rats, mice and people: Rodent biology and management. Australian Center for International Agricultural Research, Canberra.
Singleton, G.R., Jacob, J. and Krebs, C.J., 2005. Integrated management to reduce rodent damage to lowland rice crops in Indonesia. Agric. Ecosyst. Environ., 107: 75-82. https://doi.org/10.1016/j.agee.2004.09.010
Walcher, R., Karrer, J., Sachslehner, L., Bohner, A., Pachinger, B., Brandl, D., Zaller, J.G., Arnberger, A. and Frank, T., 2017. Diversity of bumblebees, heteropteran bugs and grasshoppers maintained by both: abandonment and extensive management of mountain meadows in three regions across the Austrian and Swiss Alps. Landsc. Ecol., 32: 1937-1951. https://doi.org/10.1007/s10980-017-0556-1
Witmer, G., Sayler, R., Huggins, D. and Capelli, J., 2007. Ecology and management of rodents in no-till agriculture in Washington, USA. Integr. Zool., 2: 154-64.
Yletyinen, S. and Norrdahl, K., 2008. Habitat use of field voles (Microtus agrestis) in wide and narrow buffer zones. Agric. Ecosyst. Environ., 123: 194-200. https://doi.org/10.1016/j.agee.2007.06.002
To share on other social networks, click on any share button. What are these?










