Pomegranate Juice Ameliorates Fetotoxic Effects of Atenolol in Developing Mice (Mus musculus)
Pomegranate Juice Ameliorates Fetotoxic Effects of Atenolol in Developing Mice (Mus musculus)
Sara Zafar* and Asmatullah
Department of Zoology, University of the Punjab, Quaid-i-Azam Campus, Lahore 54590, Pakistan
ABSTRACT
Our objective was to assess the fetotoxic effect of a commonly used antihypertensive drug, atenolol during gestation and ameliorative effect of pomegranate juice against its toxicity. Dose was administered to pregnant mice by gavage. The high dose contains Atenolol 2.5 µg/g body weight (B.wt.) of treated mice and low dose contains 1.65 µg/g B.wt. The dose was administered from the 6th -12th day of the gestation period for seven days. Many congenital abnormalities including hemorrhages, hygromas, resorbed fetuses, deformed, hyperextended and hyperactive flexed limbs, distorted axis, and intrauterine growth retardation was found. The present study revealed that the administration of Atenolol, especially during the organo-genetic period, can cause harm to the developing embryo and Pomegranate juice significantly reduced the detrimental effects caused by atenolol.
Article Information
Received 24 December 2018
Revised 11 May 2019
Accepted 10 June 2019
Available online 29 May 2020
Authors’ Contribution
Asmatullah planned the study and helped in histology. SZ performed experimental work and wrote the manuscript.
Key words
Gestational hypertension, Feto-toxic, Congenital abnormalities
DOI: https://dx.doi.org/10.17582/journal.pjz/20181224061217
* Corresponding author: sarazafar1133@gmail.com
0030-9923/2020/0005-1993 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
INTRODUCTION
Hypertension continues to be among the most frequent causes of maternal death during gestation (Pipkin and Roberts, 2000). Gestational hypertension further complicates about 10% of total pregnancies. Hypertension occurs when systolic blood pressure is greater than 140 mmHg and diastolic blood pressure is greater than 90 mmHg. It is accepted undoubtedly that all pregnant women with a systolic B.P of 160 mmHg or more require antihypertensive treatment (McCarthy and Kenny, 2009).
Hypertension in early pregnancy before the 7th month of gestation enhances complications in contrast to late-onset. Blood disorders were common and placental abruption was recorded at about 2.8% in the early onset of gestational hypertension (Kintiraki et al., 2015). The importance of immediate and aggressive management of high blood pressure outside pregnancy is obvious but the control of high blood pressure in pregnancy remains debatable (Easterling et al., 2001).
Antihypertensive treatment during pregnancy has increased rapidly during the past decade (Xie et al., 2013). Recent research data revealed that during pregnancy the most frequently used antihypertensive drug treatments include β adrenergic blocking agents (Andrade et al., 2008). β-blockers are particularly used as a first choice antihypertensive in young patients. These are also prescribed for young mothers suffering from gestational hypertension, to reduce hazards for the mother and the developing baby. In the USA, beta-blockers constitutes approximately 30% of all anti-hypertensive medications used in the first trimester of pregnancy (Bateman et al., 2012). Atenolol is a cardio-selective β- blocker used for the treatment of hypertension in common practice as well as in pregnancy-induced hypertension. Due to the lack of pharmacokinetic (plasma half-life of 8 hrs.) changes, atenolol is usually preferred during pregnancy (Thorley et al., 1981).
Some reports regarding atenolol fetotoxicity were published in the last few decades. Atenolol used during the gestational period resulted in intrauterine growth retardation by excessive fetal and placental vascular resistance and insufficient blood circulation in the placenta (Stephens and Wilson, 2009). These studies featured case reports where mothers were treated for hypertension. There is an absence of data with respect to the developmental toxicity potential of atenolol (Klug et al., 1994). Keeping in mind the goal to choose the most reasonable medication in the management of pregnant women with hypertensive or cardiovascular disorders, there is a requirement for more detailed information on the regenerative and toxic potential of these medications.
Pomegranate has been tested in several studies and has shown to possess a wide range of biological activities. The juice of Pomegranate was also reported for cardiotonic activity (Awari et al., 2009). Pingili and his associates confirmed the inotropic capacity of pomegranate juice on the isolated frog’s heart (Pingili et al., 2012).
This study was aimed to investigate the teratogenic properties of Atenolol. Pomegranate juice was utilized as an antidote agent in this study against damaging effects caused by atenolol during gestation. The study was carried out on mice so that the results can be translated to human beings.
MATERIALS AND METHODS
Six weeks old Swiss Webster male and female albino mice were obtained from the Veterinary Research Institute, Lahore, Pakistan, with an average body weight of about 28 ± 2. They were placed in 12”×18” sanitized shoebox steel cages (1 male: 2 female). The mice were allowed to mate freely to establish the colony. Mice were put in the animal house of Zoology Department in the University of the Punjab, where the temperature was maintained (27 ± 2 C) and good ventilation is provided. Mice were supplied with drinkable tap water in glass bottles and mouse Feed # 13 containing cereal grains supplemented with proteins, vitamins, and minerals produced by National Feeds Ltd., Lahore, Pakistan. The females mated were recognizable by the presence of white-colored vaginal plug formed by semen. That date was recorded and counted as the first day of gestation. The pregnant females were then put in isolated cages.
Dose administration
Pregnant mice were randomly divided into 7 groups: control (C), vehicle control (VC), 2 atenolol treated groups namely low dose (LD) and high dose (HD) groups and 2 Atenolol + pomegranate treated groups were made and 50% diluted pomegranate juice was provided along with each high and low doses to these groups (HD+AD), (LD+AD) from 6th_12th gestational day. One group was provided with only pomegranate juice (antidote group) to determine its effectiveness. Each group contains 10 pregnant females obtained from raised mice colony. Different concentrations of Atenolol were prepared in such a way that 0.1 ml. of each solution contained the desired amount of atenolol. Atenolol tablets were finely ground using a mortar and pestle and a Digital balance (Shimadzu Ltd Japan) was used to weigh the powdered atenolol to form the required concentration. Atenolol which is soluble in water was dissolved in distilled water at room temperature (25 ± 02).
Different concentrations of Atenolol were prepared as the high dose contains 2.5µg /g body weight (B.wt.) of treated mice and low dose contains 1.65 µg/g B.wt. The dose was administered to mice by oral gavage from 6th -12th day of gestation. Both administered doses were in the therapeutic dose range as described in previous studies. In 12 previous human studies reviewed by Sonia and her colleagues, daily atenolol doses range between 50 to 200 mg orally (Tabacova et al., 2003). While Lardoux and colleagues described human therapeutic atenolol doses range as 150±50 mg/day (Lardoux et al., 1983).
Antidote preparation and administration
Pomegranates were collected from the market, washed, and peeled by hand. The seeds obtained were crushed in a juicer. The juice extract obtained was filtered using Whatman no. 1 filter paper and then the filtrate was diluted by adding water 1:1. Each mouse approximately consumed 5ml pomegranate juice a day. Control group did not receive anything while the vehicle control group was given water orally by gavage to observe any adverse effects caused by handling and antidote group was provided with 50 % diluted pomegranate juice in drinking bottles.
All experimented females were anesthetized with chloroform and dissected at 18th gestational day and fetuses were fixed in Bouin’s fixative for at least 48 h (Carson and Hladik, 2009). The fixed embryos were transferred to 70% ethanol for further examination. For morphological analysis, binocular microscope (Labomed Ltd. Japan) was used to observe external structures. Abnormal fetuses from dose groups as well as few fetuses from control and vehicle control groups are macro-photographed using (Panasonic TZ15) digital camera to be used for explanation of fetal anomalies caused by atenolol.
Various fetuses were chosen from all the groups for histological studies. Fetuses stored in Bouin’s fixative were washed with 70% alcohol first and then were dehydrated with 70%, 90%, and 100% ethanol respectively. Fetuses were placed overnight in xylene for purpose of clearance and then for infiltration they were immersed in molten paraffin wax. Fetuses embedded in paraffin wax were subjected for cutting of 4-5µ thick transverse sections in the microtome. These sections were deparaffinized and then stained using Hematoxylin and Eosin was used for counterstaining. Microphotographs were taken by using the digital camera (Panasonic TZ15).
Statistical analysis
Morphometric analysis was done including the body weight, head circumference, eye circumference, length of fore- and hind limbs and tail length and crown-rump length of each fetus. The standard mathematical formula for calculating the circumference of an ellipse was used to calculate head circumference for each fetus in different groups.
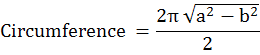
Table I. Data summary showing number of fetuses (litter size) recovered in all exposure groups.
|
Exposure group |
Control |
Vehicle control |
Pomegranate juice administered group |
Atenolol administered group |
Atenolol+Pomegranate juice administered group |
|||
|
Low |
High |
Low |
High |
|||||
|
Litter size |
102 |
97 |
101 |
99 |
84 |
99 |
94 |
|
|
Fetuses |
Normal |
102 |
97 |
101 |
82 |
51 |
75 |
64 |
|
Deformed |
0 |
0 |
0 |
15 |
25 |
11 |
13 |
|
|
No. of resorptions |
0 |
0 |
0 |
2 |
8 |
2 |
5 |
|
Table II. Statistical analysis of morphometric measurements (mm±SEM) of recovered fetuses.
|
Exposure group |
Control |
Vehicle control |
Pomeg-ranate juice admini-stered group |
Atenolol administered group |
Atenolol+ Pomegranate juice administered group |
One-way ANOVA(inter groupcompa-rison) |
|||
|
Low |
High |
Low |
High |
||||||
|
Fetal body measu-rements (mm± SEM) |
Body weight (mg±SEM) |
1236.7 ±33.47 |
1166.6± 73.38X |
1250 ±43.18 X |
894.3 ±23.86 |
655.2± 30.79 |
1007.8 ±26.38 |
991.8± 55.15 |
*** |
|
Crown rump length |
22.77 ±.20 |
21.57± .60X |
21.11 ±.40 X |
19.53 ±.36 |
16.98± .24 |
20.10 ± 27 |
19.48 ± 50 |
*** |
|
|
Eye circumference |
6.35 ±.08 |
7.28 ±.10X |
6.95± .78 X |
6.20 ±.15 |
6.21± .14 |
6.68 ± 14 |
6.38 ± 14 |
*** |
|
|
Head circumference |
22.69 ±.27 |
19.84 ±.56X |
21.33 ±.08 X |
19.71 ±.49 |
18.25 ±.19 |
19.32 ± 24 |
19.53 ± 36 |
*** |
|
|
Fore-limb length |
8.52 ±.21 |
7.86 ±.22X |
8.35± .66 X |
7.55 ±.14 |
6.39 ±.10 |
7.44 ± 08 |
7.37 ± 27 |
*** |
|
|
Hind limb length |
8.52 ±.23 |
8.69 ±.27X |
8.45± .22 X |
8.08 ±.18 |
7.00 ±.14 |
7.86 ± 18 |
8.00 ± 22 |
*** |
|
|
Tail length |
10.92 ±.14 |
9.08 ±.78X |
9.64± .33 X |
9.47 ±.25 |
8.67 ±.18 |
9.46 ± 17 |
9.82 ± 26 |
*** |
|
Asterisks show significant difference of mean values; *= p<0.05, **= p<0.01, ***= p<0.001 from control values. X = Insignificant difference of mean values from control group.
Body weights and body measurements of all the fetuses were calculated for control, Vehicle control and atenolol dose groups along with the standard error of means. Control values were used for comparison. Tukey/HSD test in combination with one way ANOVA was used for analysis.
RESULTS
Gross fetal analysis has shown that litter size was decreased in all atenolol dose groups except the Atenolol+ pomegranate-treated group where litter size was not substantially decreased. Table I describes the whole scenario produced by Atenolol administration. There is an obvious increase in resorptions and abnormal fetuses as we shift from low to high dose.
Fetuses in all experimental groups showed the variable degree of abnormalities morphologically in comparison with control and vehicle control as shown in Figure 1. All experimental groups had fetuses having abnormalities including hemorrhages, hygromas, kinked and short tail. Runt and Resorbed fetuses in uteri, distorted axis were common in the high dose group (Fig. 1A, B and C). Hemorrhages and hyperextended limbs were found in the low dose group (Fig. 1D). Hygromas and Hemorrhages were observed in high dose + pomegranate treated groups and low dose + pomegranate treated groups (Fig. 1F, G). While antidote group which only received pomegranate juice had normal and well-developed fetuses (Fig. 1E).
Histological analysis
The present study provides a detailed description of Atenolol developmental toxicity in mice. Fetal histological sections were observed for all dose groups (Control, VC, low, high, and antidote) to find any abnormalities due to atenolol in fetuses. Few sections were selected for group-wise demonstration of fetal anomalies (Fig. 2).
All fetuses from control (A) and antidote group (E) had normal features and body structures were well-developed. Low dose group had normal fetuses with few histological defects like distorted structures were noticed in a few cases (Fig. 2B). High dose group had fetuses with internal defects like misshapen and malpositioned structures with wide open spaces as shown in the nasal cavity in Figure 2C. The Atenolol + pomegranate treated groups show normal features except herniation in 4th ventricle was observed in High (Fig. 2D) dose group.
Morphometric analysis
Morphometric parameters of all the fetuses were calculated for atenolol dose groups and Atenolol+ pomegranate treated dose groups along with the standard error of means. Control values were used for intergroup comparison. Tukey/HSD test in combination with one way ANOVA was used for analysis. All parameters regarding morphometric analysis had shown a significant difference from the control group in all dose groups as well as in atenolol + pomegranate treated groups. The results for the
Vehicle control group and antidote group only treated with pomegranate juice were quite similar to the normal control group. High dose group showed more deviation from control values as compared to low dose groups whereas pomegranate treated groups had shown less deviation from control values (Table II).
The significant decrease in fetal body weight and body measurements were observed in both atenolol treated and atenolol+pomegranate treated dose groups which indicated that atenolol causes intrauterine growth retardation during gestation.
DISCUSSION
Hypertension in pregnant women does not provide a favorable condition for fetal growth and development. The risk of abortions and stillbirths is higher in hypertensive mothers while in cases where drug treatments are applied, teratological effects are recorded. In the present study, congenital malformations and intrauterine growth retardation are observed in developing mice when mothers are administered with Atenolol. Sonia and her colleagues (Tabacova and Kimmel, 2002) have reported similar results using rats and rabbits as animal models. Lennesta and colleagues (Lennestål et al., 2009) also reported the enhanced probability for placental abruption, cesarean, delivery induction and post-delivery hemorrhages in females using antihypertensive medication during pregnancy. During the study, they observed that the newborn babies were often preterm and usually were small for gestational age along with other neonatal symptoms. Atenolol specifically has been associated with fetal growth retardation (Rosenthal and Oparil, 2002) and placental changes and decreased fetal weight along with intrauterine growth retardation in neonates (Tabacova et al., 2003).
Morphometric analysis in the present study also indicates fetal growth retardation in all dose groups. The results found in this regard are comparable with previous studies.
Developmental toxicity of atenolol is assumed previously and many researchers have established similar findings in this regard (Lip et al., 1997; Lydakis et al., 1999; Tabacova and Kimmel, 2002; Tabacova Little et al., 2003). Atenolol induced fetal damage is an important concern that is needed to be resolved immediately.
Due to the ability of Atenolol to cross the placenta, it enters fetal circulation and causes harm in many ways (Thorley et al., 1981). In pregnant women taking Atenolol as antihypertensive treatment low birth weight babies (Lip et al., 1997) and high pulsatility index was observed, due to the fact that peripheral resistance in fetus and mother increased during exposure (Montan et al., 1987). This is logical to formulate comparable links between the in-utero observation in mice and human. Prenatal toxicological study of atenolol also found to have similar results in rabbit and rat species and humans (Tabacova and Kimmel, 2002).
Recent clinical trials have established numerous benefits of pomegranate juice consumption. Asgary and his associates determined that in hypertensive patient’s pomegranate juice can reduce blood pressure in about 2-2.5 weeks (Asgary et al., 2013). Pomegranate juice contains Polyphenolic flavonoids which reduced the harmful oxidative effect of low-density lipids and inhibited the development of atherosclerosis (Aviram and Rosenblat, 2012). Pomegranate juice was also reported for the presence of cardio tonic activity (Awari et al., 2009). Pomegranate juice was consumed in the study as an antidote and it considerably reduced the damaging effects of atenolol during gestation.
It can be concluded during the present study that the introduction of atenolol to the pregnant mice pose significant toxic effects in developing fetuses which can be reduced by using Pomegranate juice. Pomegranate juice not only decreased atenolol fetal toxicity but also improved maternal and fetal health. Atenolol can cause harm to the developing embryo from hemorrhages to severe deformities like Amelia and anophthalmia depending on the level of the dose administered. If the physician considers its use necessary, then likely benefits should be considered against potential threats of the drug and it is suggested that pomegranate juice should be consumed along with it to minimize the fetotoxic effects.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Andrade, S.E., Raebel, M.A., Brown, J., Lane, K., Livingston, J., Boudreau, D., Rolnick, S.J., Roblin, D., Smith, D.H., Dal-Pan, G.J., Scott, P.E. and Platt, R., 2008. Outpatient use of cardiovascular drugs during pregnancy. Pharmacoepidemiol. Drug Saf., 17: 240-247. https://doi.org/10.1002/pds.1550
Asgary, S., Mahtab K., Amirhossein, S., Mohamad, H. and Mahmoud, R,K., 2013. Clinical investigation of the acute effects of pomegranate juice on blood pressure and endothelial function in hypertensive individuals. ARYA Atheroscler., 9: 326–331.
Aviram, M. and Rosenblat, M., 2012. Pomegranate protection against cardiovascular diseases. Evid. based complement. Alternat. Med., 2012: 382763. https://doi.org/10.1155/2012/382763
Awari, D.M., Mute, V.M. and Thube B.B., 2009. Cardiotonic activity from the fruit juice of Punica granatum. J. Pharm. Res., 2: 182–184.
Bateman, B.T., Hernandez, D.S., Huybrechts, K.F., Palmsten, K., Mogun, H., Ecker, J.L. and Fischer, M.A., 2012. Patterns of outpatient antihypertensive medication use during pregnancy in a Medicaid population. Hypertension, 60: 913-920. https://doi.org/10.1161/HYPERTENSIONAHA.112.197095
Carson, F.L. and Hladik, C., 2009. Histotechnology : a self-instructional text. 3rd edn. ASCP, Chicago.
Easterling, T.R., Carr, D.B., Brateng, D., Diederichs, C. and Schmucker, B., 2001. Treatment of hypertension in pregnancy: effect of atenolol on maternal disease, preterm delivery, and fetal growth. Obstet. Gynecol., 98: 427- 433. https://doi.org/10.1097/00006250-200109000-00012
Klug, S., Thiel, R., Schwabe, R., Merker, H.J. and Neubert, D., 1994. Toxicity of β-blockers in a rat whole embryo culture: concentration-response relationships and tissue concentrations. Arch. Toxicol., 68: 375-384. https://doi.org/10.1007/s002040050085
Kintiraki, E., Papakatsika, S., Kotronis, G., Goulis, D.G. and Kotsis, V., 2015. Pregnancy-induced hypertension. Hormones (Athens), 14: 211–223. https://doi.org/10.14310/horm.2002.1582
Lardoux, H., Gerard, J., Blazquez, G., Chouty, F. and Flouvat, B.,1983. Hypertension in pregnancy: evaluation of two beta blockers atenolol and labetalol. Eur. Heart J., 4 Suppl. G.: 35–40. https://doi.org/10.1093/eurheartj/4.suppl_G.35
Lennestål, R., Olausson, P. and Källén, B., 2009. Maternal Use of antihypertensive drugs in early pregnancy and delivery outcome, notably the presence of congenital heart defects in the infants. Eur. J. clin. Pharmacol., 65: 615-625. https://doi.org/10.1007/s00228-009-0620-0
Lip, G.Y., Beevers, M., Churchill, D., Shaffer, L.M. and Beevers, D.G., 1997. Effect of atenolol on birth weight. Am. J. Cardiol., 79: 1436–1438. https://doi.org/10.1016/S0002-9149(97)00163-X
Lydakis, C., Lip, G.Y., Beevers, M. and Beevers, D.G., 1999. Atenolol and fetal growth in pregnancies complicated by hypertension. Am. J. Hyperten., 12: 541-547. https://doi.org/10.1016/S0895-7061(99)00031-X
McCarthy, F.P. and Kenny, L., 2009. Hypertension in pregnancy. Obstet. Gynaecol. Reprod. Med., 19: 136-141. https://doi.org/10.1016/j.ogrm.2009.01.001
Montan, S., Liedholm, H., Lingman, G. and Marsal, K.,1987. Fetal and uteroplacental haemodynamics during short-term atenolol treatment of hypertension in pregnancy. BJOG (an international journal of obstetrics and gynaecology), 94:312 – 317. https://doi.org/10.1111/j.1471-0528.1987.tb03097.x
Pipkin, B. and Roberts, J., 2000. Hypertension in pregnancy. J. Hum. Hyperten., 14: 705–724. https://doi.org/10.1038/sj.jhh.1001018
Pingili, R., Haroled, P.L., Ankaiah, M., Sairam, H. and Ramesh, M., 2012. Positive inotropic activity of aqueous extract of pericarp of Punica granatum on isolated frog’s heart. Int. J. Pharm. Pharm. Sci., 4: 95-98.
Rosenthal, T. and Oparil, S., 2002. The effect of antihypertensive drugs on the fetus. J. Hum. Hyperten., 16: 293–298. https://doi.org/10.1038/sj.jhh.1001400
Stephens, S. and Wilson, G., 2009. Prescribing in pregnant women : guide to general principles. Prescriber, 20: 43–46. https://doi.org/10.1002/psb.578
Tabacova, S., Little, R., Tsong, Y., Vega, A. and Kimmel, C.A., 2003. Adverse pregnancy outcomes associated with maternal enalapril antihypertensive treatment. Pharmacoepidemiol. Drug Safe., 12: 633-646. https://doi.org/10.1002/pds.796
Tabacova, S., Kimmel, C.A., Wall, K. and Hansen, D., 2003. Atenolol developmental toxicity: animal-to-human comparisons. Birth Defects Res. A Clin. Mol. Teratol., 67: 181-192. https://doi.org/10.1002/bdra.10011
Tabacova, S. and Kimmel, C., 2002. Atenolol: pharmacokinetic/dynamic aspects of comparative developmental toxicity. Reprod. Toxicol., 16: 1-7. https://doi.org/10.1016/S0890-6238(01)00193-9
Thorley, K.J., McAinsh, J. and Cruickshank, J.M., 1981. Atenolol in the treatment of pregnancy-induced hypertension. Br. J. clin. Pharmacol., 12: 725–730. https://doi.org/10.1111/j.1365-2125.1981.tb01296.x
Xie, R., Guo, Y., Krewski, D., Mattison, D., Nerenberg, K., Walker, M.C. and Wen, S.W., 2013. Trends in using beta-blockers and methyldopa for hypertensive disorders during pregnancy in a Canadian population. Eur. J. Obstet. Gynecol. Reprod. Biol., 171: 281-285. https://doi.org/10.1016/j.ejogrb.2013.09.032
To share on other social networks, click on any share button. What are these?










