Grape Infesting Mite Tetranychus urticae Koch. Resistance to Acaricides
Grape Infesting Mite Tetranychus urticae Koch. Resistance to Acaricides
Chandragouda M. Patil, Shashikant S. Udikeri* and Shreeshail. S. Karabhantanal
Department of Agricultural Entomology, College of Agriculture, Vijayapur-586101, Karnataka, India
University of Agricultural Sciences, Dharwad - 580 005, India
ABSTRACT
Studies on resistance of Tetranychus urticae Koch population from grapevine orchard indicated considerable survival on dicofol 18.5 EC, fenpyroximate 5 SC, diafenthiuron 50 SC, sulphur 80 WP, abamectin 1.9 EC, hexythiazox 5.45EC, spiromecifen 240SC, propargite 57% EC, ethion 50EC, fenazaquin 10%EC treated leaves. Field populations had high degree of resistance to Sulphur 80WG with LC 50 of 17769.72 ppm against 651.17 ppm for a laboratory susceptible strain. Thus 27.30 fold resistance ratio was observed (RR) for sulphur. The least resistance ratio 4.45 fold was observed for fenazaquin 10% EC and the field and laboratory susceptible populations have exhibited LC 50 values of 44.62 ppm and 9.57 ppm, respectively. Based on the resistance co-efficient propargite 57% EC, dicofol 18.5% EC and fenazaquin 10% EC have been classified as chemicals with low level resistance having resistance.
Article Information
Received 11 May 2018
Revised 02 May 2019
Accepted 25 September 2019
Available online 17 March 2020
Authors’ Contribution
CMP conducted the research as PG student. SSU (chairman of advisory committee) developed the research concept and facilitated the study and analyses. SSK (member of advisory committee) provided field and lab facilities and helped in analyses of data.
Key words
Tetranychus urticae, Resistance, Grape, Acaricide
DOI: https://dx.doi.org/10.17582/journal.pjz/20180511170526
* Corresponding author: udikeriss@uasd.in; ssudikeri@gmail.com
0030-9923/2020/0003-1189 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
Grape (Vitis vinifera L.) is one of the most important fruit crops of temperate zone which has acclimatized to tropical and sub tropical climatic conditions prevailing in Indian sub-continent. Grape is originated in Western Asia and Europe. It is fairly a good source of minerals like calcium, phosphorous, iron and vitamins like B1 and B2. It was introduced to India by the Persian invaders in 1300 A.D. Grape is a non-climacteric fruit that grows on the perennial and deciduous woody climbing vine.
Karnataka is the second largest grape growing state in India after Maharashtra, with an area of 20.46 thousand ha with a production of 302.39 thousand MT and productivity of 14.78 tones/ha (Anon, 2014). Grape growing regions are located in the following two agro-climatic regions in the state viz., north interior Karnataka and South interior Karnataka.
In 2014-15, Vijayapur district contributed an area of 8906 ha, and produced 1,06,536 tons of grapes, with average productivity 20 t/ha. Large acreages of grape cultivation are quite evident in Basavana bagewadi, Vijayapur, Indi, Muddebihal and Sindgi talukas of Vijayapur. Problems of viticulture in North Interior Karnataka are, i) soil andwater salinity, ii) Acute water shortage and iii) Saturation in domestic raisin market iv) Insect pests and diseases. Among non insect pests, six species of mites viz., Tetranychus urticae Koch, T. cinnabarinus Boisdual, T. neocoledonicus Andre, Oligonicus mangiferus Rahmen and Sapra, O. punicae baker and Eutetranychus orientalis Klein are found causing damage to grapevine in India (Anon., 2008). Of these mites the infestation of Tetarnychus urticae is quite considerable designating it as emerging sucking pests of grape these days (Chandra Shekhar et al., 2008). In recent years among six species, red spider mite, Tetranychus urticae Koch. (Acariformes: Tetranychidae) is causing enormous damage to grapevine in Andhra Pradesh and Karnataka. Though Tetranychus urticae is a polyphagous mite infesting many crops, the information pertaining to grapes has not been generated so far.
The problem of mite infestation has been increased a lot since last couple of years in Vijayapur district. The severity of mite menace may be due to changing pest scenario, preference of grape as a new host in the area (Veerendra et al., 2014), changing climate which is favorable for their abundant increase and heavy usage of newer pesticides which might have eliminated the natural enemies. For effective management of this pest it is essential to understand the basic causes for heavy incidence. The reasons may be resurgence and resistance linked. So, resistance study with respect to species is essential to carry out to schedule the best management practices with acaricides.
Material and methods
Present investigation on resistance of T. urtice to different acaricides was conducted in laboratory condition at College of Agriculture, Vijayapur at ambient temperature of 26±5°C and relative humidity of 74±5 per cent. The different acaricides used were Abamectin 1.9 EC, Diafenthiuorn 50 EC, Dicofol 18.5 EC, Ethion 50 EC, Fenpyroximate 5 SC, Fenazaquin 10 EC, Hexithaizox 5.45 EC, Propargite 57 EC, Spiromecifen 240 SC and Sulphur 80 WP which have been purchased as commercial products.
The field population of T. urticae was brought from grape vineyard from Dyaberi village of Vijayapura district (16042.855N, 75014.594 E,629 MSL) (Karnataka: India), collected during November-December, 2014. These field populations were reared on mulberry leaves kept upside down over sponges kept in large plastic trays containing water maintained to the surface level of sponge, so that mites were restricted only on the leaves. The leaves were changed as and when required. These mite populations were maintained in the laboratory conditions at 25± 1ºC, 70 ± 5% RH and a 14 h photoperiod. These resistant populations were reared for one generation and then used for bioassay study for ten different acaricides.
A susceptible strain of T. urticae was maintained in the laboratory conditions at 25± 1ºC, 70 ± 5% RH and 14 h photoperiod using the same methodology described for rearing the field population. The sufficient population was multiplied as required. These strains were used in determination of baseline values for susceptibility.
Baseline values are the median lethal concentration (LC50) values determined for the resistant and susceptible mite population. LC50 values were determined following FAO Leaf Dip Bioassay method. The different concentrations of different acaricides were prepared with distilled water in volumetric flasks using micropipette. The test concentrations limiting mortality to 5-95% range of different acaricides were generated through pilot studies. Within this range six to seven concentrations were used for detailed assay. Mulberry leaf discs were prepared using 25 paisa coin which makes exactly 2.0 cm diameter leaf discs. Leaf discs were dipped in desired concentration of acaricides for 5-10 seconds and exposed for 5 min to a soft current of air to eliminate excess moisture. Then leaf discs were placed adaxial side down and four leaf discs were placed in a single petri dish and remaining three were placed on other petri dish. Using a fine brush (10/0 Taklon), ten adult T. urticae females of the same age were placed on a mulberry leaf disc on water-saturated cotton (4 cm x 4 cm) in a petri dish (6 cm diameter). Water saturated cotton was pushed up against the perimeter of the leaf disc, in order to create a barrier and prevent mites from walking off the disc, since mite movement may be observed in these plates. Four replications were maintained along with a water treated control.
Observations on the mite mortality in each treatment were recorded after 24 h after treatment, which was assessed under stereo binocular microscope and mortality was worked out with corrected mortality. Mites were scored as dead if they failed to make active movement after a slight disturbance with fine brush (FAO, 1984). The mortality data were corrected using Abbot’s formula (Abbot, 1925) depending on the mortality observed in the control. The corrected mortalities were subjected Probit Analysis (Finney, 1971) using IBM SPSS Statistics version 21 for determining concentration-mortality responses and the Median Lethal concentration (LC50) values.
Abbot’s formula (Abbot, 1925):

Where, Pt = Corrected mortality;
Po = Observed mortality percentage and
Pc = Control mortality percentage.
The LC50 values determined for field populations were compared with LC50 values of susceptible laboratory culture and used for detecting and quantifying the level of resistance as Resistance Ratio.
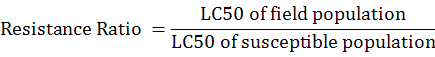
Further, the population was differentiated into categories of resistance based on resistance co-efficient as per Somnath et al. (2009) and Vaani et al. (2016).
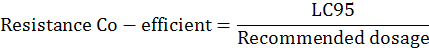
Results and discussion
The resistance has been noticed for all the ten acaricides viz., dicofol 18.5 EC, fenpyroximate 5 SC, diafenthiuron 50 SC, sulphur 80 WP, abamectin 1.9 EC, hexythiazox 5.45EC, spiromecifen 240SC, propargite 57% EC, ethion 50EC, fenazaquin 10%EC used for the study.
The median lethal concentration (LC 50) was 17,769.72 ppm for sulphur 80 WP which appeared highest among all the acaricides. The same acaricide had 651.17 ppm median lethal concentration (LC 50) for laboratory susceptible culture. Thus 27.30 fold resistance ratio was observed (RR) for sulphur. Similarly, 12.54 fold resistance (RR) was observed for ethion 50 EC where in LC 50 1,048.03 ppm was observed against 83.56 ppm for laboratory susceptible culture. All other acaricides tested had resistance ratio less than 10 fold. Among these, the least resistance ratio 4.45 fold was observed for fenazaquin 10% EC. The field and laboratory susceptible population have exhibited LC 50 values of 44.62 ppm and 9.57 ppm, respectively for fenazaquin 10% EC. The quite frequently used acaricide
Table I. Resistance in Tetranychus urticae Koch. population of grape ecosystem to different acaricides.
|
Acaricides |
Population source |
LC 50 (ppm) |
Fiducial limits |
Regression equation Y=a+bx |
x2 |
*RR values |
|
|
L L |
U L |
||||||
|
Abamectin 1.9 EC |
Field |
4.49 |
3.53 |
5.53 |
Y= 1.28+0.21x |
0.85 |
4.62 |
|
Susceptible |
0.97 |
0.40 |
1.42 |
Y= 0.70+0.37x |
0.14 |
||
|
Diafenthiuron 50 SC |
Field |
424.86 |
373.57 |
479.49 |
Y= -1.35+0.17x |
1.05 |
5.31 |
|
Susceptible |
80.023 |
54.76 |
103.32 |
Y= -3.17+2.56 x |
1.54 |
||
|
Dicofol 18.5 EC |
Field |
385.35 |
362.13 |
411.52 |
Y= -15.07+1.84x |
4.73 |
7.04 |
|
Susceptible |
54.67 |
34.23 |
83.52 |
Y= -4.39+3.91x |
3.23 |
||
|
Ethion 50 EC |
Field |
1048.03 |
942.79 |
1161.82 |
Y= -11.7+1.78 x |
4.95 |
12.54 |
|
Susceptible |
83.56 |
57.64 |
107.52 |
Y= -4.08+4.86 x |
1.44 |
||
|
Fenpyroximate 5 SC |
Field |
33.24 |
27.86 |
38.84 |
Y= -3.87+0.57x |
4.37 |
6.75 |
|
Susceptible |
4.92 |
0.061 |
8.77 |
Y= -2.13+1.51x |
0.49 |
||
|
Fenazaquin 10% EC |
Field |
42.62 |
35.55 |
49.62 |
Y= - 4.08 +0.53x |
5.44 |
4.45 |
|
Susceptible |
9.57 |
6.12 |
14.81 |
Y= - 0.77+2.05x |
5.18 |
||
|
Hexythiazox 5.45 EC |
Field |
34.18 |
27.89 |
40.02 |
Y= -3.66+0.55x |
1.37 |
4.76 |
|
Susceptible |
7.17 |
4.82 |
9.63 |
Y= -1.28+0.27x |
0.07 |
||
|
Propargite 57% EC |
Field |
604.47 |
543.27 |
664.05 |
Y= -1.63+0.18x |
3.48 |
5.37 |
|
Susceptible |
112.49 |
73.25 |
157.31 |
Y= -1.28+0.27x |
0.07 |
||
|
Spiromecifen 240 SC |
Field |
828.75 |
676.12 |
1017.41 |
Y= -6.01+0.73x |
4.25 |
5.74 |
|
Susceptible |
144.46 |
17.85 |
289.14 |
Y= -0.96+0.15x |
3.21 |
||
|
Sulphur 80 WP |
Field |
17769.72 |
16856.37 |
18742.35 |
Y= -30.51+3.78x |
4.67 |
27.30 |
|
Susceptible |
651.17 |
572.74 |
612,13 |
Y= -4.76+9.88x |
4.74 |
||
*RR, Resistance Ratio; RR, LC 50 of field population / LC 50 of susceptible population; n, 40 ( no.of mites exposed).
Table II. Acaricide resistance categories for Tetranychus urticae in grape ecosystem.
|
Acaricides |
Rec. dosage (ppm) |
LC 95 (ppm) |
Fiducial limits |
Regression equation Y=a+bx |
x2 |
Resis-tance coeffi-cient |
Rem-arks |
|
|
L L |
U L |
|||||||
|
Propargite 57% EC |
1140 |
1214.70 |
1103.78 |
1372.76 |
Y= -1.63+0.18x |
3.48 |
1.06 |
Low resist-ance |
|
Dicofol 18.5 EC |
462.50 |
719.01 |
628.38 |
885.21 |
Y= -15.07+1.84x |
4.73 |
1.55 |
|
|
Fenazaquin 10%EC |
100 |
193.06 |
146.14 |
296.78 |
Y= - 4.08 +0.53x |
5.44 |
1.93 |
|
|
Hexythiazox 5.45EC |
81.75 |
166.68 |
122.09 |
280.80 |
Y= -3.66+0.55x |
1.37 |
2.1 |
Med-ium resist-ance |
|
Diafenthiuron 50 SC |
400 |
940.46 |
827.81 |
1115.27 |
Y= -1.35+ 0.17x |
1.05 |
2.35 |
|
|
Ethion 50 EC |
1000 |
2768.71 |
2187.21 |
4212.93 |
Y= -11.77+1.78 x |
4.95 |
2.76 |
|
|
Fenpyroximate 5 SC |
50 |
147.23 |
106.33 |
255.19 |
Y= -3.87+0.57x |
4.37 |
2.94 |
|
|
Abamectin 1.9 EC |
9.50 |
30.73 |
20.97 |
56.23 |
Y= -1.28+0.21x |
0.85 |
3.23 |
|
|
Spiromecifen 240 SC |
1200 |
4201.36 |
3495.88 |
8644.66 |
Y= -6.01+0.73x |
4.25 |
3.50 |
|
|
Sulphur 80 WP |
1600 |
30115.03 |
30115.03 |
6095.04 |
Y= -30.51+3.78x |
4.67 |
18.82 |
Very high resis-tance |
[Resistance Co-efficient=LC95 /Recommended dosage] Somnath et al., 2009; Resistance Co-efficient [0.1–1.00] - lack of resistance, Resistance Co-efficient [1.1-2.0] – low resistance, Resistance Co-efficient [2.1–5.0] – medium resistance, Resistance Co-efficient [5. –10.0] – high resistance, Resistance Co-efficient [>10] – very high resistance. Population source, Field population; n, 40 (no. of mites exposed).
dicofol 18.5 EC also had LC50 of 385.35ppm in field population and 54.67 ppm for susceptible population and accounting for a resistance ratio of 7.04 fold. The teraonic and tetramic acid derivative acaricide spiromecifen had LC50 of 828.75 ppm, for field population and laboratory susceptible population had exhibited LC50 values of 144.46 and accounting for resistance ratio of 5.74 folds. Diafenthiuron had an LC50 of 424.86 ppm and 80.02 ppm for field and laboratory susceptible population, respectively and accounting for resistance ratio of 5.31 folds. The most commonly used acaricide by grape growers was Propargite 57% EC, which had an LC50 values of 604.47 ppm for field population, 112.49 ppm for susceptible population and accounting for resistance ratio of 5.37 folds. The rest of the three acaricides viz., hexythiazox 5.45 EC, fenpyroximate 5 SC, abamectin 1.9 EC had and LC 50 values of 34.18 ppm, 33.24 ppm, 4.49 ppm, respectively for field population and susceptible population had LC 50 values of 7.17 ppm, 4.92 ppm, 0.97 ppm, respectively. The RR values for these three acaricides were 4.76, 5.37 fold and 4.62 folds respectively (Table I). The present findings are in conformity with Sridhar and Jhansi Rani (2007) who reported 2-3 folds resistance to dicofol and 2 to 12 folds resistance to wettable sulphur in T. urticae populations at Delhi, Pune (Maharashtra State), Bangalore (Karnataka State) and Hosur (Tamil Nadu State).
By resistance co-efficient (Table II), propargite 57% EC, dicofol 18.5 EC and fenazaquin 10% EC have been classified as chemicals with low level resistance having resistance co-efficient in the range of 1.1 - 2. The acaricides viz., hexythiazox 5.45 EC, diafenthiuron 50 SC, ethion 50 EC, fenpyroximate 5 SC, abamectin 1.9 EC, and spiromecifen 240 SC have been classified as chemicals with medium level of resistance having resistance co-efficient in the range of 2.1–5. The most widely used acaricide as well fungicide sulphur has been classified as chemical with very high level of resistance. The exact resistance studies in grape ecosystem are not available for comparisons of the present findings. However, a few previous reports are in accordance with present findings. Young-Joon et al. (2006) has reported fenpyroximate and pyridaben resistant populations of T. urticae selected over 20 generations in the laboratory for their cross resistance to another acaricide of similar mode of action i.e., fenazaquin, the levels of resistance noticed were low (RR less than 10). Hexythiazox resistance noticed in this study is due to its poor efficacy on adult stages (Cecilia et al., 2015). Since adult females have been subjected for bioassays in the present study the resistance has been noticed. However, this acaricide has shown better efficacy against T. urticae in the grape orchards of the same locality (Veerendra et al., 2015). These mites could be effectively managed by selecting low or medium resistance category acaricides based on availability and nature of incidence. Tha acaricide like Huwa-San TR50 which has high efficacy against two spotted mites and safe to its natural predatory mites (Alhewairini and Al-Azzazy, 2018) could be tested in grape ecosystem also. Such exercise has been convenient in safflower aphid management a serious sap feeder like mites (Vaani et al., 2016). Being a resistant pest T. urticae might have experienced a serious selection pressure in grape leading resistance development through cross resistance and multiple resistance mechanism as well to keep itself un-eliminated in grape. To avoid further aggravation of the problem, regular monitoring and IRM strategies need to be implemented.
Conclusion
The widespread severe and regular infestation of mites T. urticae in grapes is due to its resistance to acaricides. The mites have high level of resistance to sulphure and are moderately resistant to many widely used acaricides. Based on resistance categories better management options are suggested for effective control.
Statement of conflict of interest
The authors declare there is no conflict of interest.
References
Abbot, W.S., 1925. J. econ. Ent., 18: 256-267. https://doi.org/10.1002/mmnd.192519250314
Alhewairini, S.S. and Al-Azzazy, M.M., 2018. Pakistan. J. Zool., 50: 241-247.
Anonymous. 2008. Management of mites on grapes, Extension folder No.15, NRC on Grapes, Pune.
Anonymous. 2014. Indian horticulture database, National Horticulture Board. www.nhb.gov.in.
Cecília, B.S., Ferreira, Fernanda H.N., Andrade, Agna, R.S., Rodrigues, Herbert, A.A., Siqueira, Manoel G.C., Gondim, Jr., 2015. Crop Protec., 67: 77-83. https://doi.org/10.1016/j.cropro.2014.09.022
Chandra-Sekhar, D., Jagadishwar, R., Rahaman, S.J., Ranga, R.A. and Narendranath, V.V., 2008. Acta Horticul., 785: 335-347. https://doi.org/10.17660/ActaHortic.2008.785.42
FAO (Food and Agriculture Organization), 1984. Pl. Prot.Bull., 32: 25-27.
Finney, D.J., 1971. Probit analysis. 3rd Ed., London, Cambridge University Press, p. 383.
Somnath, R., Mukopadhyaya A. and Gurusubramanian. 2009. Am. Euras. J. Agric. environ. Sci., 6: 244-251.
Sridhar, V. and Jhansi-Rani, B., 2007. J. Acarol., 17: 48- 50.
Vaani M.N., Udikeri S.S. and Karabhantanal, S.S., 2016. The Biosacn, 11: 841-845.
Veerendra, A.C., Udikeri, S.S. and Karabhantanal, S.S., 2014. Karnataka J. Agric. Sci., 27: 351-352.
Veerendra, A.C., Udikeri, S.S. and Karabhantanal, S.S., 2015. Int. J. Curr. Res. Biosci. Pl. Biol., 2: 130-140.
Young-Joon, K., Hyung-Man, P., Jum-Rae, C. and Young-Joon, A., 2006. J. econ. Ent., 99: 945-958. https://doi.org/10.1093/jee/99.3.954
To share on other social networks, click on any share button. What are these?










