Effectiveness of Oxytetracycline and Tylosin Phosphate on Growth of Broiler Chicken
Effectiveness of Oxytetracycline and Tylosin Phosphate on Growth of Broiler Chicken
Syed Haseeb Ahmed Shah, Irfan Shahzad Sheikh, Abdul Samad, Muhammad Kamran Taj, Mohammad Masood Tariq*, Majed Rafeeq, Sumaira Fazal, Niamatullah Kakar, Sabeera Afzal and Asadullah
Center for Advanced Studies in Vaccinology and Biotechnology (CASVAB), University of Balochitan, Quetta
ABSTRACT
Antibiotics growth promotors (AGPs) are used for enhancing the growth performance and to reduce harmful effects of bacteria in broiler chicken. These anti-microbial agents are capable of destroying the metabolism of microbes and can alter certain properties of bacterial cellular and metabolic activity which results in impaired growth or death of these bacteria. This study was planned to find out the effect of AGPs in broiler chicken. For the purpose one day old (n=432) broiler chicks were used in the experiment. Nine groups comprising of 48 chicks with 3 replicates of sixteen broiler chicks each were formed for the trial. Control group was kept on basal diet. AGPs (Oxytetracycline and Tylosin phosphate) were supplemented solely and in combinations with different concentration. Chicks were reared in floor pens bedded with saw dust litter, optimal husbandry conditions for broiler rearing were provided to the birds and vaccinations against Newcastle disease and Infectious bursal disease (IBD) were done at 9th, 19th and 11th, 23rd day, respectively. The results of current study revealed that there was non-significant difference (P>0.05) among treatment groups in feed intake and relative visceral organ weight. Whereas, a significant difference between control and AGPs supplemented groups (solely or combination) were noted in weight gain (WG), feed conversion ratio (FCR) and average daily weight gain (ADWG). Use of AGPs in combinations of OXY-1.0+TP-0.5, OXY-2.0+TP-1.0, OXY-2.0+TP-0.5, OXY-1.0+TP-1.0 had a non-significant difference (P>0.05) for WG, FCR and ADG in starter, finisher and overall rearing phases. However a significant difference (P<0.05) was observed with other groups. It can be concluded from the results of this study that supplementation of antibiotics used in broiler chicks at sub-therapeutic level is beneficial for growth performance parameters.
Article Information
Received 11 January 2021
Revised 11 March 2021
Accepted 25 March 2021
Available online 15 June 2021
(early access)
Published 26 February 2022
Authors’ Contribution
SHAS, ISS, SF and MMT conducted the research and collected the data. SA, MMT and AS, analyze the data. NK, Asadullah and MR carried out setting the paper and writing the manuscript.
Key words
Antibiotic growth promotors, Broiler chicken, Oxytetracycline, Tylosin.
DOI: https://dx.doi.org/10.17582/journal.pjz/20210111110158
* Corresponding author: tariqkianiraja@hotmail.com
0030-9923/2022/0003-1313 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
Introduction
Broiler meat is an essential product to provide safe, economical and superior quality protein for human diet. Antibiotics are biological ingredients that are formed naturally by a microorganism, which are used for inhibiting the growth of other microbes. These anti-microbial agents destroy the metabolism of microbes they are very useful to inhibit the growth of bacteria. An antibiotic can alter certain properties of cellular and metabolic activity of bacteria which results in impaired growth or death. At poultry farms, antibiotics are used as a source of improving the growth, health and immunity against different harmful bacteria (Sahu, 2016). The strength of the gastrointestinal tract (GIT) and microbiota present in gut plays a significant role in the utilization of nutrients, enhancement of immunity, and development of resistance against diseases in the broilers (Kheiri et al., 2018). Since long, poultry industry has been using anti-microbial agents to improve meat production through better feed efficiency, to gain better growth rate, to fight against bacterial infections (Mehdi et al., 2018). Supplementing broilers with antibiotic growth promotors (AGPs) at sub-therapeutic levels in their diet increases 3.3-8.0% of gain and improve approximately 3% of feed efficiency (FE). Anti-microbial spectrum, anti-microbial activity, anti-microbial mechanism, and even toxicity to normal cells of different anti-microbial peptides have great differences (Wang et al., 2020). Since these AGPs have long been in practice as growth promoters, still its need of time that AGPs should be investigated for their safer use as growth promotor in broiler chicken feeds (Choi et al., 2018). Beneficial bacteria such as Lactobacillus enhances the growth ultimately improving health of broiler chicken while harmful bacteria such as Salmonella and Campylobacter cause diseases and suppress the growth (Hillman et al., 2017; Torok et al., 2011). The beneficial impact of combination therapy applied to a broader antibacterial spectrum, for getting their synergistic effects (Ahmed et al., 2014), to reduce risk for severe sepsis and septic shock during therapy (Tangden et al., 2014; Kumar et al., 2010). These combinations result in reduced mortality rate as compared to the individual antibiotic (Baddour et al., 2004; Engberg et al., 2000).
Materials and Methods
Research Centre
This study was completed at Center of Advance Studies in Vaccinology and Biotechnology (CASVAB), University of Balochistan in Quetta on broiler chicken.
Husbandry of broiler chicks
The trial was conducted on broiler chicken for single In-vivo experiment. Day old broiler chicks were received from International poultry, Karachi, Pakistan sub office Multan. The chicks were kept under recommended management conditions. Daily feed allowance was supplemented with antibiotic growth promotors (AGPs) solely and in combinations as different treatments. Pre-starter feeding (F-1) was given from day 1st to day 12th, starter feed (F-2) was offered from day 13th to 24th and finally finisher feed was provided from day 25th till the end of experiment (42nd day). Fresh ad libitum drinking water was offered to the chicks. According to the husbandry protocols, for the 1st week after arrival of chicken the shed temperature was maintained at 95°F and after that the temperature was reduced 5°F weekly. The bird’s vaccination against New castle disease and Infectious bursal disease (IBD) were done at 9th, 19th and 11th, 23rd day, respectively.
AGPs supplementations
Four hundred and thirty two (n=432) broiler chicks were used in the experiment. Nine groups of broiler chicks were formed comprising of 48 chicks in each group having 3 replicates of sixteen broiler chicks each. Control group was kept on basal diets (without any AGPs), other supplemented with AGPs. Detail of treatments with dose is given in Table I. Oxytetracycline and Tylosin were used because of their low cost, availability and either not or very meager usage in human being.
Duration of trial
The trial was completed in 6 weeks on broiler chickens.
Parameters
Growth performance
Data regarding growth performance parameters such as daily weight gain (WG), feed intake (FI), average daily weight gain (ADG) and feed conversion ratio (FCR) was collected from 1st to 6th week of rearing. Slaughtering of birds was done on 14th, 28th and 42nd day for relative visceral organ weight (Sheikh et al., 2020).
Weight gain (WG)
Weight gain of broiler chicken was recorded using digital weighing balance with accuracy of ± 100 g from 1st week to 6th week separately. For distinct treatment group, data was evaluated for starter phase (1-21 days), finisher phase (22-42 days) and overall growth period (1-42 days).
Feed intake (FI)
Daily calculated but restricted feed were provided in the trial. On day 1 chick were provided crushed corn. Feeding was started @6-grams/day/chick and simultaneously increased @ (3-5) grams/day/chick. Data was analyzed simultaneously for (starter phase) 1st to 3rd week, (finisher phase) 4th to 6th week and (overall rearing phase) 1st to 6th week by given formulae:
FI per bird = Feed given (g) - Feed consumed (g)
Feed conversion ratio (FCR)
Feed conversion ratio (FCR) indicates better economic performance in production of broiler chicks. The purpose of feed conversion ratio is to attain the maximum body weight with minimum cost of feed. The relationship of mathematical calculation between weight gained and
Table I.- Supplementation of antibiotic growth promotors.
|
S. No |
Groups |
Treatments |
Dose |
|
1 |
Control |
Basal diet |
Without AGPs |
|
2 |
OXY-1.0+TP-0 |
Oxytetracycline Di-hydrate |
100 mg per kg of feed |
|
3 |
OXY-0+TP-0.5 |
Tylosin Phosphate |
50 mg per kg of feed |
|
4 |
OXY-1.0+TP-0.5 |
Oxytetracycline Di-hydrate + Tylosin Phosphate |
100 mg per kg of feed + 50 mg per kg of feed |
|
5 |
OXY-2.0+TP-0 |
Oxytetracycline Di-hydrate |
200 mg per kg of feed |
|
6 |
OXY-0+TP-1.0 |
Tylosin Phosphate |
100 mg per kg of feed |
|
7 |
OXY-2.0+TP-1.0 |
Oxytetracycline Di-hydrate + Tylosin Phosphate |
200 mg per kg of feed + 100 mg per kg of feed |
|
8 |
OXY-2.0+TP-0.5 |
Oxytetracycline Di-hydrate + Tylosin Phosphate |
200 mg per kg of feed + 50 mg per kg of feed |
|
9 |
OXY-1.0+TP-1.0 |
Oxytetracycline Di-hydrate +Tylosin Phosphate |
100 mg per kg of feed + 100 mg per kg of feed |
feed consumed of a bird was attained on 3rd, 6th and 1to 6th week of trail by formulae given below:
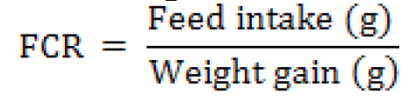
Average daily weight gain (ADG)
Average daily weight gain (grams) of distinct group was observed on weekly basis. Data was recorded simultaneously for 1st to 3rd week, 4th to 6th week and 1st to 6th week by given formulae. Average daily weight gain (ADG) was also noted for starter phase, finisher and weight gain overall by using formula:
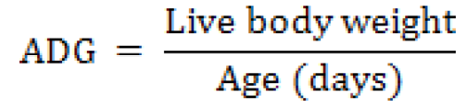
Carcass percentage (CP)
At 14th, 21st and 42nd day three 3 broiler chicks/group were slaughtered to find out the percentage (%) of carcass.
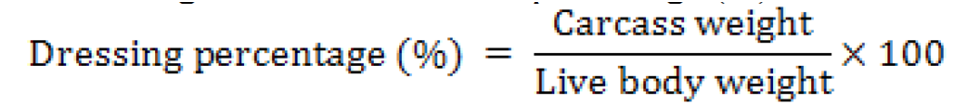
Relative visceral organ weight (RVOW)
At the day 14th, 28th and 42nd, three 3 broiler chickens from each group were slaughtered to find out the relative visceral organ weight (%). Relative visceral organ weights (RVOW) e.g. heart, proventriculus, liver, gizzard, and relative intestinal lengths were calculated using formulae mention below:
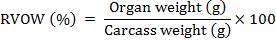
Statistical analysis
The data was organized in Microsoft Excel. Statistical analysis was carried out using SPSS 21 analysis of variance (ANOVA) with interaction technique, followed by post hoc Duncan multiple range (DMR) test where needed.
Results
The outcomes of supplementing these antibiotic growth promoters in different phases of rearing of broiler chicken showed better growth performance with respect to weight gain, improved FCR and average daily gain.
Feed intake
As far as total feed consumed in this trial was concerned there were non-significant differences (P˃0.05) in feed intake (FI 1-3) for starter phase, finisher phase (FI 4-6) and at 42 days (FI 1-6) of rearing (Table II).
Table II.- Feed intake (FI) (g) in different phases (1-3, 4-6 and 1-6 weeks) of broiler rearing (Mean±SE).
|
Treatments |
FI 1-3 |
FI 4-6 |
FI 1-6 |
|
Control |
865.33±3.76 |
2856.00±5.86 |
3721.33±8.67 |
|
OXY-1.0+TP-0 |
857.00±3.06 |
2858.00±5.00 |
3715.00±3.79 |
|
OXY-0+TP-0.5 |
849.00±5.29 |
2861.33±15.38 |
3710.3±18.70 |
|
OXY-1.0+TP-0.5 |
863.00±7.64 |
2861.33±17.17 |
3724.33±16.22 |
|
OXY-2.0+TP-0 |
850.67±6.64 |
2872.33±2.19 |
3723.00±7.21 |
|
OXY-0+TP-1.0 |
864.33±3.18 |
2849.33±19.88 |
3713.67±19.22 |
|
OXY-2.0+TP-1.0 |
851.33±2.40 |
2871.33±14.05 |
3722.67±11.67 |
|
OXY-2.0+TP-0.5 |
850.67±7.13 |
2848.00±15.71 |
3698.73±16.79 |
|
OXY-1.0+TP-1.0 |
850.67±4.97 |
2859.33±14.72 |
3710.00±18.33 |
|
P-value |
P>0.05 |
P>0.05 |
P>0.05 |
abcmeans with different superscripts in each column shows significant difference (P<0.05) .
Weight gain
The results of growth performance during starter, finisher and overall rearing phase showed a significant difference (P<0.05) in weight gain (Table III).
Table III.- Weight gain (WG) (g) in different phases (1-3, 4-6 and 1-6 weeks) of broiler rearing (Mean±SE).
|
Treatments |
WG 1-3 |
WG 4-6 |
WG 1-6 |
|
Control |
685.95±3.05c |
1730.89±4.01c |
2416.84±6.29c |
|
OXY-1.0+TP-0 |
710.06±5.13b |
1756.86±6.64b |
2466.93±11.54b |
|
OXY-0+TP-0.5 |
714.20±1.87b |
1757.63±1.30b |
2471.84±3.17b |
|
OXY-1.0+TP-0.5 |
749.21±2.82a |
1786.90±7.15a |
2536.11±7.41a |
|
OXY-2.0+TP-0 |
714.82±3.09b |
1760.27±3.60b |
2475.09±4.53b |
|
OXY-0+TP-1.0 |
715.33±3.48b |
1759.63±5.93b |
2474.96±3.49b |
|
OXY-2.0+TP-1.0 |
757.05±0.48a |
1791.74±2.38a |
2548.79±2.49a |
|
OXY-2.0+TP-0.5 |
748.10±0.23a |
1778.8±8.44a |
2526.91±8.65a |
|
OXY-1.0+TP-1.0 |
750.06±2.04a |
1784±3.21a |
2564.53±2.3a |
|
P-value |
P<0.05 |
P<0.05 |
P<0.05 |
abcmeans with different superscripts in each column shows significant difference (P<0.05).
Feed conversion ratio
The results of feed conversion ratio (FCR 1-3) for starter, finisher (FCR 4-6) and overall rearing period (FCR 1-6) in 42 days of rearing revealed that there were significant differences (P˂0.05) between control and AGPs supplemented groups (Table IV).
Average daily weight gain
The outcomes obtained by supplementation of Oxytetracycline and Tylosin Phosphate in all treatment groups for average daily weight gain has been elaborated in Table V. The results of average daily weight gain (ADG 1-3) for starter phase, average daily weight gain for finisher
Table IV.- Feed conversion ratio (FCR) (g) in different phases (1-3, 4-6 and 1-6 weeks) of broiler rearing (Mean±SE).
|
Treatments |
FCR 1-3 |
FCR 4-6 |
FCR 1-6 |
|
Control |
1.26±0.00c |
1.65±0.01c |
1.54±0.00c |
|
OXY-1.0+TP-0 |
1.21±0.01b |
1.62±0.01b |
1.51±0.01b |
|
OXY-0+TP-0.5 |
1.19±0.01b |
1.63±0.01b |
1.50±0.01b |
|
OXY-1.0+TP-0.5 |
1.15±0.01a |
1.60±0.01a |
1.47±0.00a |
|
OXY-2.0+TP-0 |
1.19±0.01b |
1.63±0.00b |
1.50±0.01b |
|
OXY-0+TP-1.0 |
1.21±0.01b |
1.62±0.02b |
1.50±0.01b |
|
OXY-2.0+TP-1.0 |
1.12±0.00a |
1.60±0.01a |
1.46±0.01a |
|
OXY-2.0+TP-0.5 |
1.13±0.01a |
1.60±0.00a |
1.46±0.00a |
|
OXY-1.0+TP-1.0 |
1.13±0.15a |
1.60±0.00a |
1.46±0.00a |
|
P-value |
P<0.05 |
P<0.05 |
P<0.05 |
abcmeans with different superscripts in each column shows significant difference (P<0.05).
Table V.- Average daily weight gain (ADG) (g) in different phases (1-3, 4-6 and 1-6 weeks) of broiler rearing (Mean±SE).
|
Treatments |
ADG 1-3 |
ADG 4-6 |
ADG 1-6 |
|
Control |
32.66±0.15c |
82.42±0.19c |
57.55±0.15c |
|
OXY-1.0+TP-0 |
33.81±0.25b |
83.66±0.31b |
58.74±0.27b |
|
OXY-0+TP-0.5 |
34.01±0.09b |
83.69±0.06b |
58.85±0.08b |
|
OXY-1.0+TP-0.5 |
35.68±0.14a |
85.09±0.34a |
60.39±0.18a |
|
OXY-2.0+TP-0 |
34.04±0.15b |
83.82±0.17b |
58.93±0.11b |
|
OXY-0+TP-1.0 |
34.06±0.17b |
83.79±0.28b |
58.93±0.08b |
|
OXY-2.0+TP-1.0 |
36.05±0.03a |
85.32±0.11a |
60.69±0.06a |
|
OXY-2.0+TP-0.5 |
35.62±0.01a |
84.70±0.40a |
60.16±0.20a |
|
OXY-1.0+TP-1.0 |
35.71±0.10a |
84.97±0.15a |
60.34±0.05a |
|
P-value |
P<0.05 |
P<0.05 |
P<0.05 |
abcmeans with different superscripts in each column shows significant difference (P<0.05).
Table VI.- Relative organ and carcass weight (%) at 14th, 28th and 42nd day of rearing of broiler rearing (Mean±SE).
|
Day |
Treatments |
Carcass |
Liver |
Heart |
Prov |
Gizzard |
Spleen |
Int-length |
|
14th day |
Control |
55.82±1.07 |
9.21±0.51 |
1.84±0.14 |
1.27±0.16 |
6.45±0.12 |
0.39±0.04 |
24.00±0.39 |
|
OXY-1.0+TP-0 |
52.99±2.47 |
11.42±1.32 |
2.04±0.15 |
1.42±0.07 |
5.44±0.41 |
0.40±0.09 |
25.96±0.87 |
|
|
OXY-0+TP-0.5 |
51.57±3.32 |
8.23±0.25 |
1.93±0.18 |
1.39±0.13 |
5.84±0.61 |
0.26±0.04 |
28.23±1.86 |
|
|
OXY-1.0+TP-0.5 |
52.48±4.26 |
10.39±2.10 |
2.26±0.28 |
1.27±0.18 |
5.63±1.16 |
0.28±0.14 |
27.82±2.98 |
|
|
OXY-2.0+TP-0 |
50.03±6.40 |
11.15±1.71 |
2.20±0.33 |
1.21±0.08 |
5.81±0.48 |
0.33±0.08 |
27.11±2.83 |
|
|
OXY-0+TP-1.0 |
53.25±2.04 |
12.31±0.93 |
1.88±0.13 |
1.34±0.12 |
5.53±0.24 |
0.47±0.03 |
28.50±0.92 |
|
|
OXY-2.0+TP-1.0 |
56.06±1.32 |
11.53±2.04 |
2.21±0.16 |
1.40±0.22 |
6.23±1.26 |
0.48±0.08 |
27.18±1.54 |
|
|
OXY-2.0+TP-0.5 |
53.18±1.50 |
11.94±0.34 |
1.80±0.19 |
1.30±0.08 |
5.58±0.68 |
0.45±0.05 |
27.28±0.86 |
|
|
OXY-1.0+TP-1.0 |
55.03±1.30 |
11.89±2.26 |
1.85±0.22 |
1.22±0.11 |
5.97±0.78 |
0.39±0.02 |
27.32±1.31 |
|
|
28th day |
Control |
59.58±0.72 |
6.59±0.12 |
1.20±0.06 |
0.84±0.03 |
4.00±0.65 |
0.33±0.03 |
8.68±0.34 |
|
OXY-1.0+TP-0 |
62.03±2.12 |
6.28±0.21 |
1.07±0.07 |
0.85±0.04 |
4.00±0.15 |
0.32±0.03 |
8.16±0.49 |
|
|
OXY-0+TP-0.5 |
60.42±3.03 |
6.51±0.48 |
1.19±0.01 |
0.87±0.03 |
4.64±0.07 |
0.33±0.04 |
8.85±0.27 |
|
|
OXY-1.0+TP-0.5 |
56.94±1.09 |
6.41±0.26 |
1.18±0.01 |
0.91±0.05 |
4.14±0.18 |
0.31±0.02 |
9.07±0.25 |
|
|
OXY-2.0+TP-0 |
59.90±1.56 |
6.42±0.31 |
1.12±0.09 |
0.84±0.04 |
4.32±0.17 |
0.31±0.03 |
8.48±0.31 |
|
|
OXY-0+TP-1.0 |
59.82±1.79 |
6.29±0. 28 |
1.27±0.02 |
0.99±0.08 |
4.73±0.57 |
0.29±0.03 |
8.60±0.07 |
|
|
OXY-2.0+TP-1.0 |
59.71±1.73 |
6.84±0.46 |
1.25±0.12 |
0.96±0.04 |
4.86±0.37 |
0.31±0.04 |
9.15±0.39 |
|
|
OXY-2.0+TP-0.5 |
60.05±1.25 |
5.92±0.76 |
1.23±0.08 |
0.96±0.08 |
4.42±0.54 |
0.29±0.03 |
8.55±0.06 |
|
|
OXY-1.0+TP-1.0 |
59.34±1.67 |
6.47±0.74 |
1.28±0.14 |
0.96±0.07 |
4.88±0.36 |
0.31±0.02 |
9.02±0.40 |
|
|
42nd day |
Control |
61.27±0.23 |
4.35±0.07 |
0.98±0.06 |
0.75±0.07 |
4.18±0.73 |
0.30±0.06 |
5.16±0.25 |
|
OXY-1.0+TP-0 |
62.02±0.22 |
4.29±0.13 |
0.92±0.06 |
0.75±0.15 |
3.66±0.17 |
0.32±0.11 |
4.89±0.31 |
|
|
OXY-0+TP-0.5 |
61.42±0.34 |
4.19±0.32 |
0.79±0.09 |
0.81±0.04 |
4.75±0.25 |
0.37±0.04 |
5.30±0.34 |
|
|
OXY-1.0+TP-0.5 |
61.34±0.12 |
4.37±0.07 |
0.98±0.03 |
0.77±0.07 |
3.50±0.29 |
0.40±0.01 |
4.65±0.34 |
|
|
OXY-2.0+TP-0 |
61.38±0.37 |
4.11±0.18 |
0.85±0.10 |
0.66±0.03 |
4.10±0.46 |
0.32±0.07 |
4.86±0.20 |
|
|
OXY-0+TP-1.0 |
61.17±0.59 |
4.04±0.07 |
0.82±0.09 |
0.77±0.06 |
4.44±0.64 |
0.32±0.02 |
5.00±0.11 |
|
|
OXY-2.0+TP-1.0 |
61.40±0.08 |
4.57±0.11 |
0.99±0.03 |
0.87±0.08 |
3.92±0.44 |
0.38±0.01 |
4.71±0.20 |
|
|
OXY-2.0+TP-0.5 |
60.43±0.29 |
4.03±0.08 |
1.01±0.12 |
0.73±0.01 |
4.41±0.58 |
0.33±0.04 |
4.97±0.17 |
|
|
OXY-1.0+TP-1.0 |
60.85±0.91 |
4.20±0.36 |
0.89±0.12 |
0.83±0.13 |
3.86±0.41 |
0.36±0.02 |
4.80±0.20 |
|
|
P-value |
P>0.05 |
P>0.05 |
P>0.05 |
P>0.05 |
P>0.05 |
P>0.05 |
P>0.05 |
abcmeans with different superscripts in each column shows significant difference (P<0.05).
phase (ADG 4-6) and average daily weight gain (ADG 1-6) in 42 days of rearing revealed that there were significant differences (P<0.05) in average daily weight gain between control, Single supplemented antibiotic and in combination antibiotic in all three phases of rearing. The results revealed better average daily weight gain when these antibiotic growth promoters were offered in combinations (Table V).
Relative visceral organ weight
Oxytetracycline and tylosin phosphate were provided solely or in combination with different concentrations/levels to broiler chicken from 1st to 42nd days (6 weeks). The outcomes of supplementing these antibiotic growth promoters at 14th, 28th and 42nd day of relative organ weight rearing of broiler chicken (Table VI). Effect of antibiotic growth promotors on visceral organs weight (carcass, liver, heart, proventriculus, gizzard, spleen and intestinal-length) showed a non-significant difference (P˃0.05) between control, single supplemented antibiotic and in combination antibiotic in all three phases of rearing.
Supplementation of oxytetracycline at 1% and 2% to the basal diet showed significant improvement (P<0.05) in weight gain, feed conversion ratio and average daily weight gain of broiler chicken during starter, finisher and overall rearing period (Table VII).
Table VII.- Effect of oxytetracycline (OXY) on growth performance of broiler chicken during starter, finisher and whole rearing period (Mean±SE).
|
Parameters |
OXY 0 |
OXY 1.0% |
OXY 2.0% |
|
Starter phase (1-21 days) |
|||
|
FI (g) |
859.56±3.01 |
856.89±3.01 |
850.89±3.01 |
|
WG (g) |
705.16±1.65b |
736.44±1.65a |
739.99±1.65a |
|
FCR (g) |
1.22±0.00b |
1.17±0.00a |
1.15±0.00a |
|
ADG (g) |
33.58±0.08b |
35.07±0.08a |
35.24±0.08a |
|
Finisher phase (22-42 days) |
|||
|
FI (g) |
2856±7.82 |
2860±7.82 |
2864±7.82 |
|
WG (g) |
1750±3.03b |
1776±3.03a |
1777±3.03a |
|
FCR (g) |
1.63±0.00b |
1.61±0.00a |
1.61±0.00a |
|
ADG (g) |
83.30±0.14b |
84.57±0.14a |
84.61±0.14a |
|
Whole rearing period (1-42 days) |
|||
|
FI (g) |
2856±7.82 |
2860±7.82 |
2864±7.82 |
|
WG (g) |
2455±3.63b |
2512±3.63a |
2517±3.63a |
|
FCR (g) |
2.85±0.00b |
2.34±0.00a |
1.91±0.00a |
|
ADG (g) |
58.44±0.08b |
59.82±0.08a |
59.92±0.08a |
abcmeans with different superscripts in each column shows significant difference (P<0.05).
Supplementation of tylosin phosphate at 0.5% and 1% to the basal diet showed significant improvement (P<0.05) in weight gain, feed conversion ratio and average daily weight gain of broiler chicken during starter, finisher and overall rearing period (Table VIII).
Table VIII.- Effect of tylosin phosphate (TP) on growth performance of broiler chicken during starter, finisher and whole rearing period (Mean ±SE).
|
Parameters |
TP 0 |
TP 0.5% |
TP 1.0% |
|
Starter phase (1-21 days) |
|||
|
FI (g) |
857.67±3.01 |
854.22±3.01 |
855.44±3.01 |
|
WG (g) |
703.61±1.65b |
737.17±1.65a |
740.81±1.65a |
|
FCR (g) |
1.22±0.00b |
1.16±0.00a |
1.16±0.00a |
|
ADG (g) |
33.51±0.08b |
35.10±0.08a |
35.28±0.08a |
|
Finisher phase (22-42 days) |
|||
|
FI (g) |
2862±7.82 |
2857±7.82 |
2860±7.82 |
|
WG (g) |
1750±3.03b |
1774±3.03a |
1780±3.03a |
|
FCR (g) |
1.63±0.00b |
1.61±0.00a |
1.60±0.00a |
|
ADG (g) |
83.30±0.14b |
84.49±0.14a |
84.69±0.14a |
|
Whole rearing period (1-42 days) |
|||
|
FI (g) |
3720±8.34 |
3711±8.34 |
3715±8.34 |
|
WG (g) |
2453±3.63b |
2512±3.63a |
2519±3.63a |
|
FCR (g) |
2.85±0.00b |
2.34±0.00a |
1.91±0.00a |
|
ADG (g) |
58.40±0.08b |
59.80±0.08a |
59.98±0.08a |
abcmeans with different superscripts in each column shows significant difference (P<0.05).
Supplementation of oxytetracycline at 1% and 2% to the basal diet showed non-significant (P>0.05) in carcass, liver, heart, proventriculus, gizzard, spleen and Intestinal length of broiler chicken during starter, finisher and overall rearing period (Table XI).
Supplementation of tylosin phosphate at 0.5% and 1% to the basal diet showed non-significant difference (P>0.05) in Carcass, liver, heart, proventriculus, gizzard, spleen and intestinal length of broiler chicken during starter, finisher and overall rearing period (Table X).
Discussion
Broiler chicken has a very short life span with rapid growth. AGPs are used for enhancing the growth performance and to reduce harmful bacterial load in broiler chicken. These anti-microbial agents are capable of destroying the metabolism of microbes and can alter certain properties of bacterial cellular and metabolic activity which results in impaired growth or death of these
Table IX.- Effect of oxytetracycline on visceral organs and carcass percentage of broiler chicken at 14th, 28th and 42nd day of rearing (Mean ±SE).
|
Day |
Organ |
OXY 0 |
OXY 1.0% |
OXY 2.0% |
|
14th day |
Carcass |
53.55±1.80 |
53.50±1.80 |
53.09±1.80 |
|
Liver |
9.92±0.85 |
11.24±0.85 |
11.54±0.85 |
|
|
Heart |
1.89±0.12 |
2.05±0.12 |
2.07±0.12 |
|
|
Prov |
1.34±0.08 |
1.30±0.08 |
1.30±0.08 |
|
|
Gizzard |
5.94±0.42 |
5.68±0.42 |
5.87±0.42 |
|
|
Spleen |
0.42±0.06 |
0.36±0.06 |
0.48±0.06 |
|
|
Int-lenght |
26.91±1.00 |
27.03±1.00 |
27.19±1.00 |
|
|
28th day |
Carcass |
59.93±1.02 |
59.43±1.02 |
59.88±1.02 |
|
Liver |
6.46±0.26 |
6.38±0.26 |
6.39±0.26 |
|
|
Heart |
1.21±0.04 |
1.17±0.04 |
1.20±0.04 |
|
|
Prov |
0.90±0.02 |
0.90±0.02 |
0.92±0.02 |
|
|
Gizzard |
4.45±0.22 |
4.34±0.22 |
4.53±0.22 |
|
|
Spleen |
0.31±0.01 |
0.31±0.01 |
0.30±0.01 |
|
|
Int-lenght |
8.71±0.18 |
8.75±0.18 |
8.72±0.18 |
|
|
42nd day |
Carcass |
61.28±0.24 |
61.29±0.24 |
61.07±0.24 |
|
Liver |
4.19±0.10 |
4.28±0.10 |
4.23±0.10 |
|
|
Heart |
0.86±0.04 |
0.93±0.04 |
0.95±0.04 |
|
|
Prov |
0.77±0.04 |
0.78±0.04 |
0.75±0.04 |
|
|
Gizzard |
4.45±0.27 |
3.67±0.27 |
4.14±0.27 |
|
|
Spleen |
0.39±0.0 |
0.34±0.0 |
0.33±0.0 |
|
|
Int-lenght |
5.15±0.14 |
4.78±0.14 |
4.84±0.14 |
abcmeans with different superscripts in each column shows significant difference (P<0.05).
bacteria. As far as the results of present experiment is concerned, significant difference (P<0.05) in average weight gain, FCR and average daily gain was noted with AGPs in broiler chicken. The mechanism involved in antibiotic growth promoters on improved growth performance of farm animals might be due to the decrease in pathogen load, reduction of microbial metabolites, reduction of nutrients loss in the gut and enhanced uptake of nutrients due to improved intestinal histological status (Brussow, 2015). Withdrawal of AGPs may cause a rapid increase in the microbial population in the gut that will ultimately affect its production performance. It is estimated that about 6-10% of the nutrient supplied in the feed are consumed by gut micro flora at the same time these microbes cause pathological conditions in the birds (Peinado et al., 2012; Jimoh et al., 2013). In the absence of antimicrobial substances birds have to combat with the harmful effect of microbes and their metabolic products. This competition causes utilization of nutrients for the protection rather than utilization for the production.
Table X.- Effect of tylosin phosphate (TP) on visceral organs and carcass percentage of broiler chicken at 14th, 28th and 42nd day of rearing (Mean±SE).
|
Day |
Organ |
TP 0 |
TP 0.5% |
TP 1.0% |
|
14th day |
Carcass |
52.95±1.80 |
52.41±1.80 |
54.78±1.80 |
|
Liver |
10.59±0.85 |
10.19±0.85 |
11.91±0.85 |
|
|
Heart |
2.03±0.12 |
2.00±0.12 |
1.98±0.12 |
|
|
Prov |
1.30±0.08 |
1.32±0.08 |
1.32±0.08 |
|
|
Gizzard |
5.90±0.42 |
5.68±0.42 |
5.91±0.42 |
|
|
Spleen |
0.42±0.06 |
0.33±0.06 |
0.50±0.06 |
|
|
Int-lenght |
25.69±1.00 |
27.78±1.00 |
27.67±1.00 |
|
|
28th day |
Carcass |
60.50±1.02 |
59.13±1.02 |
59.62±1.02 |
|
Liver |
6.43±0.26 |
6.27±0.26 |
6.53±0.26 |
|
|
Heart |
1.13±0.04 |
1.20±0.04 |
1.26±0.04 |
|
|
Prov |
0.84±0.02 |
0.91±0.02 |
0.97±0.02 |
|
|
Gizzard |
4.10±0.22 |
4.40±0.22 |
4.82±0.22 |
|
|
Spleen |
0.32±0.01 |
0.31±0.01 |
0.30±0.01 |
|
|
Int-lenght |
8.44±0.18 |
8.82±0.18 |
8.92±0.18 |
|
|
42nd day |
Carcass |
61.44±0.24 |
61.06±0.24 |
61.14±0.24 |
|
Liver |
4.25±0.10 |
4.19±0.10 |
4.26±0.10 |
|
|
Heart |
0.91±0.04 |
0.92±0.04 |
0.90±0.04 |
|
|
Prov |
0.71±0.04 |
0.77±0.04 |
0.82±0.04 |
|
|
Gizzard |
3.97±0.27 |
4.21±0.27 |
4.07±0.27 |
|
|
Spleen |
0.31±0.03 |
0.36±0.03 |
0.35±0.03 |
|
|
Int-lenght |
4.96±0.14 |
4.97±0.14 |
4.83±0.14 |
abcmeans with different superscripts in each column shows significant difference (P<0.05).
Non-significant difference (P>0.05) of feed intake during starter, finisher and overall growth phases was found in control and AGPs treated groups. Hussein et al. (2020) in their studies also concluded that supplementation of AGPs in feed had non-significant (P>0.01) effect on feed consumption in broiler chicken as compared to non-supplemented group (control). Identical results were also reported by other researches (Silva et al., 2018; Tayeri et al., 2018). Current study is in line with the findings of Khadem et al. (2014) that also reported non-significant difference in feed intake using Oxytetracycline as AGP.
Gilani et al. (2018) found non-significant variations in the concentration of the liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum between control and AGP treated groups with boost in weight gain. Whereas hemoglobin, lymphocytes, neutrophils, eosinophils, monocytes, basophils and platelets levels at 21 and 42 days of rearing were found normal blood physiological ranges of broiler chicken. Cardinal et al. (2020) determined that AGPs supplemented feed broiler groups had gained significantly (P<0.05) faster growth and higher weight gain as compared to basal diet fed group. These findings were also in agreement with the results of present studies. Other researchers (Tayeri et al., 2018; Kalavathy et al., 2008) also reported beneficial effect of AGPS on growth parameters. Similarly, Hussein et al. (2020) reported significantly (P<0.01) better growth rate of different supplemented groups of AGPs at day 35th of broiler chicks rearing. Contrary to the findings of this study, Hamid et al. (2019) reported that AGP incremented groups had non-significant (P>0.05) results in growth performance of broiler chicken. These differences in the results might be due to dosage level used in that research.
The findings of this study revealed that AGPs supplemented groups had better FCR then the control group from 1-3, 4-6 and 1-6 weeks. These results were in line with the finding of Khadem et al. (2014) who reported significant improvement (P<0.05) in FCR in response of AGPs supplementation in broiler chickens at day 21st and 35th. The findings of Cardinal et al. (2020) also showed significantly (P<0.05) enhanced feed conversion ratio with the usage of AGPs. Similarly outcomes of Hamid et al. (2019) research revealed that FCR significantly (P<0.05) improved by using chlortetracycline as compare to basal in-fed group. Haque et al. (2017) investigation were also in accord with the outcomes of existing study where improved FCR was obtained in those groups which were incremented with AGPs. Significantly (P<0.05) higher FCR (1.83) of feed was also reported with antibiotics supplementation (Tayeri et al., 2018). Kalavathy et al. (2008) revealed that Oxytetracycline fed groups had significantly (P<0.05) better FCR than that of basal diet group at 42nd day of rearing in broiler chicken. However a few researchers (Hussein et al., 2020; Silva et al., 2018) didn’t report any effect (P>0.05) on feed conversion ratio in AGPs supplemented groups.
In the current study AGPs supplemented groups had significantly (P<0.05) higher average daily weight gain in comparison to control group in starter, finisher and overall experiment. The outcomes of this study were in accordance with the results of He et al. (2019). Similarly, Cardinal et al. (2020) reported that AGPs treatments had significantly (P<0.05) better daily weight gain as compared to non-supplemented group. Silva et al. (2018) concluded that AGPs supplemented groups had improved daily weight gained from 1 to 42 days of rearing these findings were also in line with outcomes of present study. The results of Hamid et al. (2019) were not in agreement with the outcomes of current study because AGPs supplemented groups didn’t show any influence (P>0.05) on the growth performance and daily weight gain it might be due to the types and dose of AGPs used in both trials.
Supplementation of AGPs mainly suppresses gut microbial growth and their metabolites, but also absorbed and metabolized in the liver. Roshanfekr and Mamooee (2009) reported better growth performance in AGPs treated groups as compared to control, prebiotic and probiotic groups without effecting blood physiological parameters. ALT and AST are released from the liver cells into the bloodstream. When damage occurs in liver, any abnormal increase in their concentration can indicate liver malfunction and poor growth (Attia et al., 2011; Bhatti and Dil, 2005).
Conclusion
In conclusion, oxytetracycline and tylosin (AGPs) supplementation in feed, improved growth of broiler chickens when given solely or in combination as compared to control group. AGPs significantly enhanced the weight gain, feed conversion ratio, and average daily weight gain (ADG) during starter, finisher, and overall rearing phase. Overall better results were noted in treatment group 4 supplemented combinations of both antibiotics. There was no interaction effect on the performance of broiler was noted when AGPs were given in combination. AGPs had no effect on the feed intake and relative visceral organ weight. It can be suggested that group 4 is economically feasible combination for the broiler chicken and low dosage may cause lower side effect.
Statement of conflict of interest
The authors have declared no conflict of interests.
References
Ahmed, A., Azim, A., Gurjar, M. and Baronia, A.K., 2014. Current concepts in combination antibiotic therapy for critically ill patients. Indian J. Crit. Care Med., 18: 310. https://doi.org/10.4103/0972-5229.132495
Attia, Y.A., Zeweil, H.S., Alsaffar, A.A. and El-Shafy, A.S., 2011. Effect of non-antibiotic feed additives as an alternative to flavomycin on productivity, meat quality and blood parameters in broilers. Arch. Geflügelk, 75: 40-48.
Baddour, L.M., Yu, V.L., Klugman, K.P., Feldman, C., Ortqvist, A., Rello, J. and Chiou, C.C., 2004. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am. J. Respirat. Crit. Care med., 170: 440-444. https://doi.org/10.1164/rccm.200311-1578OC
Bhatti, B.M. and Dil, S., 2005. Effect of vitamin C on immune response in desi chicken against newcastle disease. Pakistan J. Vet. Res., 2: 48-49.
Brüssow, H., 2015. Growth promotion and gut microbiota: Insights from antibiotic use. Environ. Microbiol., 17: 2216-2227. https://doi.org/10.1111/1462-2920.12786
Cardinal, K.M., da Silva Pires, P.G. and Ribeiro, A.M.L., 2020. Growth promoter in broiler and pig production. Med. Vet. Zootec., 14: 1-11.
Choi, J.H., Lee, K., Kim, D.W., Kil, D.Y., Kim, G.B. and Cha, C.J., 2018. Influence of dietary avilamycin on ileal and cecal microbiota in broiler chickens. Poult. Sci., 97: 970-979. https://doi.org/10.3382/ps/pex360
Danzeisen, J.L., Kim, H.B., Isaacson, R.E., Tu, Z.J. and Johnson, T.J., 2011. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One, 6: 0027949. https://doi.org/10.1371/journal.pone.0027949
Engberg, R.M., Hedemann, M.S., Leser, T.D. and Jensen, B.B., 2000. Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poult. Sci., 79: 1311-1319. https://doi.org/10.1093/ps/79.9.1311
Gilani, S.M.H., Zehra, S., Galani, S. and Ashraf, A., 2018. Effect of natural growth promoters on immunity, and biochemical and haematological parameters of broiler chickens. Trop. J. Pharmaceut. Res., 17: 627-633. https://doi.org/10.4314/tjpr.v17i4.9
Hamid, H., Zhao, L.H., Ma, G.Y., Li, W.X., Shi, H.Q., Zhang, J.Y. and Ma, Q.G., 2019. Evaluation of the overall impact of antibiotics growth promoters on broiler health and productivity during the medication and withdrawal period. Poult. Sci., 98: 3685-3694. https://doi.org/10.3382/ps/pey598
Haque, M.I., Ahmad, N. and Miah, M.A., 2017. Comparative analysis of body weight and serum biochemistry in broilers supplemented with some selected probiotics and antibiotic growth promoters. J. Adv. Vet. Anim. Res., 4: 288-294. https://doi.org/10.5455/javar.2017.d226
He, T., Long, S., Mahfuz, S., Wu, D., Wang, X., Wei, X. and Piao, X., 2019. Effects of probiotics as antibiotics substitutes on growth performance, serum biochemical parameters, intestinal morphology, and barrier function of broilers. Animals, 9: 985. https://doi.org/10.3390/ani9110985
Hillman, E.T., Lu, H., Yao, T. and Nakatsu, C.H., 2017. Microbial ecology along the gastrointestinal tract. Microb. Environ., 32: 300-313. https://doi.org/10.1264/jsme2.ME17017
Hussein, E.O., Ahmed, S.H., Abudabos, A.M., Aljumaah, M.R., Alkhlulaifi, M.M., Nassan, M.A. and Swelum, A.A., 2020. Effect of antibiotic, phytobiotic and probiotic supplementation on growth, blood indices and intestine health in broiler chicks challenged with Clostridium perfringens. Animals, 10: 507. https://doi.org/10.3390/ani10030507
Jimoh, A.A., Ibitoye, E.B., Dabai, Y.U. and Garba, S., 2013. In vivo antimicrobial potentials of garlic against Clostridium perfringens and its promotant effects on performance of broiler chickens. Pakistan J. biol. Sci., 16: 1978-1984. https://doi.org/10.3923/pjbs.2013.1978.1984
Kalavathy, R., Abdullah, N., Jalaludin, S., Wong, C.M.V.L. and Ho, Y.W., 2008. Effect of Lactobacillus cultures and oxytetracycline on the growth performance and serum lipids of chickens. Int. J. Poult. Sci., 7: 385-389. https://doi.org/10.3923/ijps.2008.385.389
Khadem, A., Soler, L., Everaert, N. and Niewold, T.A., 2014. Growth promotion in broilers by both oxytetracycline and Macleaya cordata extract is based on their anti-inflammatory properties. Br. J. Nutr., 112: 1110-1118. https://doi.org/10.1017/S0007114514001871
Kheiri, F., Faghani, M. and Landy, N., 2018. Evaluation of thyme and ajwain as antibiotic growth promoter substitutions on growth performance, carcass characteristics and serum biochemistry in Japanese quails (Coturnix japonica). Anim. Nutr., 4: 79-83. https://doi.org/10.1016/j.aninu.2017.09.002
Kumar, A., Zarychanski, R., Light, B., Parrillo, J., Maki, D. and Simon, D., 2010. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: A propensity-matched analysis. Crit. Care Med., 38: 1773-1785. https://doi.org/10.1097/CCM.0b013e3181eb3ccd
Mehdi, Y., Létourneau-Montminy, M.P., Gaucher, M.L., Chorfi, Y., Suresh, G., Rouissi, T. and Godbout, S., 2018. Use of antibiotics in broiler production: Global impacts and alternatives. Anim. Nutr., 4: 170-178. https://doi.org/10.1016/j.aninu.2018.03.002
Peinado, M.J., Ruiz, R., Echávarri, A. and Rubio, L.A., 2012. Garlic derivative propyl propane thiosulfonate is effective against broiler enteropathogens in vivo. Poult. Sci., 91: 2148-2157. https://doi.org/10.3382/ps.2012-02280
Roshanfekr, H. and Mamooee, M., 2009. Effect of dietary antibiotic, probiotic and prebiotic as growth promoters, on growth performance, carcass characteristics and hematological indices of broiler chickens. Pak. J. biol. Sci., 12: 52-57. https://doi.org/10.3923/pjbs.2009.52.57
Sahu, H.S., 2016. Effect of antibiotic growth promoters on quantitative morphology and histology of broiler. Pre-Ph D Course Work, Submitted to North Orissa University.
Sheikh, I.S., Bajwa, M.A., Rashid, N., Mustafa, M.Z., Tariq, M.M., Rafeeq, M. and Ullah, A., 2020. Effects of immune modulators on the immune status of broiler chickens. Pakistan J. Zool., 52: 1095. https://doi.org/10.17582/journal.pjz/20190519110533
Silva, G.V.D., Machado, N.D.J.B., Freitas, L.W.D., Lima, M.F.D. and Luchese, R.H., 2018. Performance and carcass yield of female broilers fed with diets containing probiotics and symbiotics as an alternative to growth enhancers. Acta Sci. Anim. Sci., 40: e39916. https://doi.org/10.4025/actascianimsci.v40i1.39916
Tängdén, T., 2014. Combination antibiotic therapy for multidrug-resistant Gram-negative bacteria. Upsala J. med. Sci., 119: 149-153. https://doi.org/10.3109/03009734.2014.899279
Tayeri, V., Seidavi, A., Asadpour, L. and Phillips, C.J., 2018. A comparison of the effects of antibiotics, probiotics, synbiotics and prebiotics on the performance and carcass characteristics of broilers. Vet. Res. Commun., 42: 195-207. https://doi.org/10.1007/s11259-018-9724-2
Torok, V.A., Allison, G.E., Percy, N.J., Ophel-Keller, K. and Hughes, R.J., 2011. Influence of antimicrobial feed additives on broiler commensal posthatch gut microbiota development and performance. Appl. environ. Microbiol., 77: 3380-3390. https://doi.org/10.1128/AEM.02300-10
Wang, G., Song, Q., Huang, S., Wang, Y., Cai, S., Yu, H. and Zhang, J., 2020. Effect of antimicrobial peptide microcin J25 on growth performance, immune regulation, and intestinal microbiota in broiler chickens challenged with Escherichia coli and Salmonella. Animals, 10: 345. https://doi.org/10.3390/ani10020345
To share on other social networks, click on any share button. What are these?









