Distribution, Dietary Breadth and Niche Overlap between Two Sympatric Mongoose Species Inhabiting Pir Lasura National Park, Azad Jammu and Kashmir, Pakistan
Distribution, Dietary Breadth and Niche Overlap between Two Sympatric Mongoose Species Inhabiting Pir Lasura National Park, Azad Jammu and Kashmir, Pakistan
Faraz Akrim1,2, Tariq Mahmood1,*, Muhammad Sajid Nadeem3, Siddiqa Qasim1, Shaista Andleeb1 and Hira Fatima1
1Department of Wildlife Management, PMAS-Arid Agriculture University, Rawalpindi
2Department of Zoology, University of Kotli, Azad Jammu and Kashmir
3Department of Zoology, PMAS-Arid Agriculture University, Rawalpindi
ABSTRACT
Knowledge of a predator’s diet and distribution is vital for its conservation and management. We investigated dietary breadth of two carnivore species; Indian grey mongoose (Herpestes edwardsii) and small Indian mongoose (Herpestes javanicus) occurring in the north-eastern Himalayan region (Azad Jammu and Kashmir) of Pakistan with a view to compute the niche overlap between the two species. The Indian grey mongoose was recorded at 15 different sampling sites within the elevation range 699-1559 m above mean sea level (AMSL). The small Indian mongoose was found distributed at 30 different sites in the study area, having an elevation range 691-1624 m AMSL. The diet of Indian grey mongoose consisted of 16 prey species (15 wild and one domestic), and six plant species. The consumption of wild prey was 60%, while domestic prey contributed 19%, plants 14%, grits 2%, and anthropogenic matter (plastic bags, and threads) 5%. In comparison, 17 dietary items were recorded in the diet of small Indian mongoose, including 10 wild, 1 (one) domestic and 6 plant species. Consumption of wild prey was 60%, domestic prey 17% plant matter 11%, grits 2% and anthropogenic matter ~10%. Dietary niche breadth of Indian grey mongoose was found broad (0.83) during autumn season but narrow (0.36) during winter season. On the other hand, the dietary niche breadth of small Indian mongoose was found broad during summer season 0.59 but narrow during spring season 0.46. The two sympatric mongoose species overlapped (0.89) in their dietary niche breadth in the study area.
Article Information
Received 18 May 2018
Revised 23 July 2018
Accepted 13 February 2019
Available online 15 May 2019
Authors’ Contribution
FA, TM and MSN conceptualized the study and wrote manuscript. FA conducted the field and lab work. SQ, SA and HF helped in data analysis.
Key words
Sympatric carnivores, Indian grey mongoose, Small Indian mongoose, Diet composition, Niche overlap.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.4.1497.1507
* Corresponding author: tariqjanjua75@uaar.edu.pk
0030-9923/2019/0004-1497 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Indian grey mongoose Herpestes edwardsii, a small carnivore, is mainly found in south Asia; Pakistan, Afghanistan, India, Nepal up to Ceylon (now Sri Lanka). The species also occupies coastal areas of Saudi Arabia and Iran (Ewer, 1973; Nowak, 2005; Francis, 2008; Gilchrist et al., 2009). It has also been introduced to Japan and Peninsular Malaysia (Francis, 2008; Gilchrist et al., 2009). In Pakistan, it is common in central and northern parts of Sindh province particularly inhabiting the desert tracts of Tharparkar. It also occurs in some parts of the Punjab; Rawalpindi and the Salt Range. In Balochistan, grey mongoose is sparsely found in the southern parts. It also occurs in Peshawar, Kohat, Buner and Bannu districts in the province of Khyber Pakhtunkhwa (Roberts, 1997; Akhtar et al., 2018).
The small Indian mongoose Herpestes javanicus, another small carnivore, occurs in Asia (South and south-eastern part) (Wozencraft, 2005), where it has a native range from Pakistan to northern India, southern part of China and the Malay Peninsula. It has been reported from Hainan Island, Java and southern Iran (Corbet and Hill, 1992), Afghanistan (Hassinger, 1968), Kuwait and Iraq (Harrison, 1968). In Pakistan, small Indian mongoose is distributed in Sindh, Punjab and Balochistan. In Punjab, it has been reported from Lahore, Kasur, Sialkot, Gujranwala and Jhelum districts. It also occurs in the Salt Range and sparsely in Bahawalpur division. It has not yet been reported from Khyber Pakhtunkhwa (Roberts, 1997).
Mammalian scats have been commonly used in biological studies to estimate population size (Kohn et al., 1999; Webbon et al., 2004), distribution patterns or species richness (Dalén et al., 2004), diet composition (Mahmood et al., 2011) as they are abundant and easily found (Sanz et al., 2007). Faecal components of carnivores can comprise of feathers, bones, hairs, teeth, claws, scales, arthropod chitin, plant matter, mucus cells, and bacteria (Bang and Dahlström, 1975; Bujne, 2000), whereas the quantity and size of carnivore scats can be different based on age of individuals, prey species consumed and absorption capacity (Bang and Dahlström, 1975).
Scats can be used for animal identification (Seton, 1925; Camardella et al., 2000), activity centers of animals (Walker, 1996), composition of diet (Chinchilla, 1997; Santos and Hartz, 1999; Kauhala and Auniola, 2001), seasonal changes in diet (Aragona and Setz, 2001), prey species inventory (Camardella et al., 2000), role in seed dispersal (Fragoso and Huffman, 2000; Williams et al., 2000), animal health condition, and entero-parasitosis dynamics (Page et al., 2001).
Knowledge of a predator’s diet is vital to understand its ecology and to predict its effect on the dynamics of prey populations (Oli, 1993). Despite widespread occurrence of mongooses across Azad Jammu and Kashmir (AJ&K), Pakistan, no scientific information is available on diet composition of mongooses though data from other regions are available (Roberts, 1997). To improve our understanding of vital ecological parameters, we, in the current study, investigated diet composition and seasonal variation in diet, dietary niche breadth and niche overlap of two different mongoose species in and around Pir Lasura National Park (NP), Azad Jammu and Kashmir, Pakistan (Fig. 1).
Materials and methods
Study area
The current study was carried out in and around Pir Lasura National Park (PLNP) Tehsil Nakyal, District Kotli, Azad Jammu and Kashmir, Pakistan (Fig. 1). The study area is located in the south-eastern part of AJ&K, close to the Line of Control between 33°25.92 N to 33°29.31 N and 74°05.64 E to 74°03.02 E. The National Park encompasses 1580 ha area with elevation ranging between 1000–2000 m above mean sea level (AMSL) (Akrim et al., 2018a). The valleys in the NP support subtropical pine forest type vegetation. The climate of the study area is cold and humid. The temperature of study area ranges between -7 to 50°C during the year. The average annual rainfall is 1500 mm (Akrim et al., 2017, 2018b).
The major wildlife species of the study area include: common leopard Panthera pardus, red fox (Vulpes vulpes), Asiatic jackal (Canis aureus), Indian pangolin (Manis crassicaudata), rhesus monkey (Macaca mulatta), barking deer (Muntiacus muntjak), and kalij pheasant (Lophura leucomelanos).
Distribution of two sympatric mongoose species
Extensive field surveys were conducted to document distribution of the two sympatric mongoose species in the study area from 2014 to 2017, by recording direct (direct sightings and road killed animals) and indirect signs like scats of the two species following Wemmer et al. (1996). Information about occurrence of mongooses was also collected from local community living in the vicinity of the study area and field staff of the department of Fisheries and Wildlife AJ&K. Data on site, geographic location, elevation, date and species identification for each scat were recorded, and processed in Quantum GIS (Version 2.2.3) and Arc GIS (Version 10.1) to produce distribution maps.
Diet composition
Diet composition of the two mongoose species occurring in the study area was investigated by analysis of their scat samples. We conducted surveys to collect scats of the two carnivore species during 2014-2016 using area searches technique. All scats were collected from outside of burrows and activity areas of each mongoose species in the study area. When a scat was encountered, the field identification was determined based on its morphology including diameter, length, shape, color, odor, physical appearance such as characteristic contents (hairs, bones and plant material) (Seton, 1925; Jackson and Hunter, 1995). Additional criteria included nature of scat deposit site, and presence of tracks or signs of activity of the species under study.
Scat analysis
The scat samples were correctly assigned to each of the mongoose species, for diet analysis. The scat samples were sun dried and morphological characteristics of scats such as length, breadth and weight recorded.
For disintegration, scat samples were soaked in warm water and then washed under tap water in a sieve to remove dust and mucus and segregated different prey items such as hairs, bones, insects, bird feathers and plant parts (Mahmood et al., 2013). The prey parts were dried and divided into different groups such as plant-based diet, and animal-based diet. The weight of each dietary item such as hairs, bones, feathers, insects and plant parts was recorded using electronic weighing balance to compute percent volume.
Whole mount preparation
We used hairs for identification of mammalian prey species. For this purpose, slides of hairs of prey species were prepared. Hairs were washed in carbon tetrachloride for 15-20 min. Long hairs were cut into small pieces and jumbled up hairs were separated. For whole mount preparation, we used transparent nail polish.
Prey species of mongoose species were identified using medullary pattern and cuticle cast pattern of hairs recovered from scat samples as described by Moore et al. (1974). Prepared slides were then compared with reference hair slides for identification. Similarly, other parts recovered from scat samples were also identified such as bones, bird feathers. Invertebrates such as insects and plant matter including seeds. The hairs of prey species were identified using Light microscope, having objective lenses of 10x, 40x and 100x magnification.
Scale replication
Cuticular scale patterns of mammalian hair were identified by slightly modifying the procedure of Lavoie (1971). Two to three drops of transparent nail polish were placed and spread evenly on glass slide. A small hair was placed in vertical position along axis of slide so as one end of hair projected out of slide. After the nail polish was dry the end of hair projecting out was plucked with a single attempt using forceps to get cast of hair on nail polish. The cast of hair prepared was the exact duplicate of scales of the hair and was studied under microscope against reference for identification.
Identification of plant matter recovered
Plant matter recovered from scats of mongoose species mainly comprised of seeds and parts of fruit. Recovered seeds and fruit remains were compared with reference material collected from the field and identified. Seeds were also sown in pots to germinate for plant species identification.
Dietary niche breadth
We measured dietary niche breadth of two sympatric mongoose species using niche breadth (L) and standardized Levins index (0-1) (Lst) (Levins, 1968; Colwell and Futuyma, 1971) as follows:
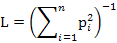
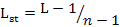
Where pi is the relative percentage of food item i and n is the number of food items.
Lst is standardized niche breadth and its value ranges from 0 to 1. A higher Lst indicates broader diet niche of the animal.
Niche overlap
We used the frequency of occurrence of each prey item to compute dietary overlap between the two sympatric mongoose species occurring in the study area using Pianka’s index, the value of which ranges from zero (no overlap) to one (complete overlap) (Pianka, 1973). We chose this index to allow direct comparison of the degree of overlap in similar studies of carnivores conducted elsewhere in the world (Fedriani et al., 2000; Ray and Sunquist, 2001; Jacomo et al., 2004). The Pianka’s index was calculated using the formula:
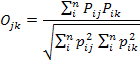
Where pij (or pik) is the relative percentage of food item i in diet j (or k).
The Pianka’s index ranges from 0 to 1 and the higher value indicates higher degree of overlap in diet.
The prey species diversity index (H’), prey richness (S) and prey evenness (E) indices were calculated for each of the two mongoose species during different seasons. Prey species richness (S) is the total number of prey species consumed by each predator during each season.
Diversity index (H’) was calculated by using the following formula:
H’ = -Σ [pi × ln pi]
Where, pi is the prey index.
The evenness index (E) was calculated by using the formula:
E = H’/ln of S
Where, S represents the prey species richness and H’ represents the diversity index.
Statistical analysis
To estimate abundance of mongoose species using their signs, we used a Kernel density analysis. The Kernel density analysis expresses sign density of a species (including direct and indirect signs) per kilometer square of study area. Mapping kernel density allowed us to identify areas having high sign density of mongoose species. To estimate a density value, we used bandwidth of 2000m as a search radius to calculate sign abundance of mongoose species per square kilometer in the 20m cell. This 2000m radius is logical and reasonable to identify priority areas where conservation measures should be taken. Estimated density values were then classified using Jenks methods (Jenks and Caspall, 1971).
Statistically, data were analyzed using SPSS (version 23) software (SPSS Inc., Chicago, USA) and Excel statistics. We compared total frequency of dietary items consumed by each mongoose species for statistical differences. To compare seasonal variation in diet composition of each mongoose species we used generalized linear model (GLM). Similarly, we compared seasonal variation in consumption of wild prey species, domestic prey species, and plant matter. We repeated GLM for variation in consumption of each dietary item consumed by each mongoose species.
Results
Distribution
The Indian grey mongoose was recorded at fifteen different sampling sites with an elevation range 699–1559 m AMSL. Scats of the Indian grey mongoose (n = 57) were collected from five different study sites which included; GDC Nakyal (n = 8; 14%), Katera (n = 14; 24.5%), Nakyal (n = 19; 33.3%), Pothi Sairi (n = 5; 8.8%), and Supply (n = 11; 19.3%). The direct field sightings of the species were recorded at fourteen different sites (Fig. 1). While a high sign density of Indian grey mongoose was found at Nakyal sampling site in the study area (Fig. 2).
The small Indian mongoose was found distributed at thirty different sites in the study area, within an elevation range 691-1624 m AMSL. Scats of small Indian mongoose were found and collected from seven different sampling sites including Katera (n = 6; 8.7%), Kothian (n = 10; 14.5%), Panagali (n = 8; 11.6%), Pir Kana (n = 17; 24.6%), Pothi Sairi (n = 4; 5.8%), Sairi (n = 6; 8.7%) and Supply (n =18; 26%). The species was directly field sighted at eighteen sites; road killed individuals of the small Indian mongoose were encountered at four sites, while indirect sightings of the mongoose species were reported at eleven different sites (Fig. 3). A high density of small Indian mongoose signs was estimated at Pir Kana, Sairi, Katera and Supply sampling sites (Fig. 4).
Diet composition
Indian grey mongoose
Analysis of 57 scat samples showed that 22 prey species occurred in the diet of Indian grey mongoose. Among them 15 animal species were from the wild, one domestic and six plant species. Diet of Indian grey mongoose consisted of wild species (60%), domestic prey species (19%), plant species (14%), grit (2%), and anthropogenic matter (5%). Among wild prey species consumption of house mouse Mus domesticus was high and the only domestic prey species i.e., chicken/poultry accounted for (19%). Among plant species consumption of phitni bairi Zizyphus oxyphylla was high (5%) (Table I; Fig. 5). General linear model explained 64.7% variation in consumption of dietary items by Indian grey mongoose (R squared=0.647). The variation in consumption of dietary items was highly significant t (F= 5.74, df = 23, p = 0.000).
Seasonal variation in diet composition of Indian grey mongoose was assessed by conducting analysis of 57 samples; 13 in summer, 18 in autumn, 14 in winter and 12 in spring. Consumption of wild prey species was high during summer season (72%) and low during spring season (52.63%). Consumption of domestic prey species was high during winter season (29.17%) and low during summer season (8%). Consumption of plant species was high during summer season (16%) and low during winter season (8.33%). General linear model (GLM) showed that diet of Indian grey mongoose did not differ significantly during four seasons F= 1.028, df=3, p=0.38. Consumption of wild prey species did not differ significantly F=0.53, df=3, p=0.66. Similarly, consumption of plant species did not differ significantly F=0.37, df=3, p=0.77 (Table I).
Small Indian mongoose
Analysis of 69 scat samples of small Indian mongoose showed that 17 prey species occurred in its diet. Among them 10 were wild prey species, only one domestic prey species and six plant species. Frequency of occurrence of wild prey was (59.68%), domestic prey (16.94%), plant matter (11.29%), grit (2.4%), and anthropogenic matter (9.7%). Among wild prey species frequency of occurrence of house rat Rattus rattus was high (10.48%) followed by house mouse (9.68%). The only domestic species i.e., hen/poultry accounted for 16.94%. Among plants frequency of occurrence of Jaro grass (Themeda anathera) was high (4.03%) followed by phitni bairi (2.42%) (Table II; Fig. 5). GLM explained 67.1% variation in the diet of small Indian mongoose. There was significant difference in consumption of different dietary items (F= 6.61, df= 18, p=0.000).
Among 69 scats, 17 were collected during summer season, 21 in autumn, 12 during winter season and 19 during spring season. Consumption of wild prey was high during summer season (66.67%) and low during winter season (54.17%). Consumption of domestic prey was high during spring season (24.24%) and low during summer season (10%). Consumption of plant species was high
Table I.- Percent frequency (% F) of occurrence of prey items in the diet of Indian grey mongoose in Pir Lasura National Park, Azad Jammu and Kashmir Pakistan.
|
Prey species/items recovered |
Summer (n=13) |
Autumn (n=18) |
Winter (n=14) |
Spring (n=12) |
% Total frequency |
|
House mouse (Mus musculus) |
16 |
12.5 |
12.5 |
5.26 |
12 |
|
Indian gerbil (Tatera indica) |
12 |
12.5 |
4.17 |
10.53 |
10 |
|
Norway rat (Rattus norvegicus) |
4 |
3.13 |
4.17 |
5.26 |
4 |
|
Roof or house rat (Rattus rattus) |
0 |
3.13 |
12.5 |
0 |
4 |
|
Spotted dove (Streptopelia chinensis) |
4 |
0 |
4.17 |
5.26 |
3 |
|
Himalayan bulbul (Pycnonotus leucogenys) |
4 |
0 |
0 |
0 |
1 |
|
House sparrow (Passer domesticus) |
4 |
3.13 |
0 |
5.26 |
3 |
|
Red-vented bulbul (Pycnonotus cafer) |
0 |
3.13 |
0 |
0 |
1 |
|
Reptiles |
4 |
3.13 |
0 |
5.26 |
3 |
|
Amphibians |
0 |
3.13 |
0 |
0 |
1 |
|
Orthoptera (grasshopper) |
4 |
3.13 |
12.5 |
5.26 |
6 |
|
Hymanoptera (ants and bees) |
12 |
9.38 |
4.17 |
5.26 |
8 |
|
Coleoptera (beetles) |
4 |
0 |
0 |
0 |
1 |
|
Snail (Cornu spp.) |
0 |
3.13 |
0 |
0 |
1 |
|
Egg shells |
4 |
0 |
0 |
5.26 |
2 |
|
Total wild prey |
72 |
59.38 |
54.17 |
52.63 |
60 |
|
Domestic prey |
|||||
|
Poultry birds (Gallus gallus domesticus) |
8 |
15.63 |
29.17 |
26.32 |
19 |
|
Total domestic prey |
8 |
15.63 |
29.17 |
26.32 |
19 |
|
Plants |
|||||
|
Chhoti bairi fitni (Ziziphus oxyphylla) |
12 |
6.25 |
0 |
0 |
5 |
|
Water melon (Citrullus lanatus) |
4 |
6.25 |
0 |
0 |
3 |
|
Apple (Pyrus malus) |
0 |
3.13 |
0 |
5.26 |
2 |
|
Dhania (Coriandrum sativum) |
0 |
0 |
4.17 |
0 |
1 |
|
Jaru grass (Themeda anathera) |
0 |
0 |
4.17 |
5.26 |
2 |
|
Khabbal grass (Cynodon dactylon) |
0 |
0 |
0 |
5.26 |
1 |
|
Total plants |
16 |
15.63 |
8.33 |
15.79 |
14 |
|
Grit |
0 |
3.13 |
4.17 |
0 |
2 |
|
Anthropogenic |
4 |
6.25 |
4.17 |
5.26 |
5 |
during summer season (13.33%) and low during autumn season (8.1%). GLM showed that diet of small Indian mongoose did not differ significantly during four seasons F=0.38, df = 3, p = 0.76. Consumption of wild prey did not differ significantly F = 0.138, df = 3, p = 0.93. Similarly, consumption of plant species during four seasons was not statistically significant F=0.094, df = 3, p = 0.96.
Niche breadth and niche overlap
Niche breadth of Indian grey mongoose was broad 20 (0.83) during autumn season but narrow 9.3 (0.36) during winter season. Overall, niche breadth of Indian grey mongoose was 18 (0.72). Dietary niche breadth of small Indian mongoose was found broad during summer season 11.64 (0.59) but narrow during spring season 9.35 (0.46). Total niche breadth of small Indian mongoose was 12 (0.64) (Fig. 6).
Overall, the niche overlap between small Indian mongoose and the Indian grey mongoose was estimated to be 0.89.
Prey species indices
Prey species diversity in the diet of Indian grey mongoose was high during autumn season 2.63 and relatively low during winter season 2.2. Prey species richness was high during autumn season (17) and low during winter season (12). Prey species evenness was high during summer and spring season 0.94 each and low during winter season 0.89. Prey species diversity index in the diet of small Indian mongoose was high during autumn season 2.48 and low during winter 2.23. Prey species richness was high during autumn and spring 14 species and low in winter 11 species. Prey species evenness was high during summer 0.95 and low during spring 0.90) (Fig. 7).
Table II.- Percent frequency (%F) of occurrence of prey items in the diet of small Indian mongoose in Pir Lasura National Park, Azad Jammu and Kashmir, Pakistan
|
Prey species/items recovered |
Summer (n=17) |
Autumn (n=21) |
Winter (n=12) |
Spring (n=19) |
% Total frequency |
|
Wild prey species |
|||||
|
Indian Gerbil (Tatera indica) |
6.67 |
5.4 |
4.17 |
3.03 |
4.82 |
|
House mouse (Mus musculus) |
13.33 |
5.4 |
12.5 |
9.09 |
9.68 |
|
Roof or house rat (Rattus rattus) |
10 |
13.51 |
8.33 |
9.09 |
10.48 |
|
House sparrow (Passer domesticus) |
3.33 |
0 |
0 |
3.03 |
1.61 |
|
Red-vented bulbul (Pycnonotus cafer) |
0 |
0 |
4.17 |
3.03 |
1.61 |
|
Amphibians |
3.33 |
8.1 |
0 |
0 |
3.23 |
|
Reptiles |
6.67 |
2.7 |
0 |
6.06 |
4.03 |
|
Orthoptera (grasshopper) |
16.67 |
10.81 |
16.67 |
6.06 |
12.1 |
|
Hymenoptera (ants, bees) |
6.67 |
8.1 |
4.17 |
15.15 |
8.87 |
|
Coleoptera (beetles) |
0 |
5.4 |
4.17 |
3.03 |
3.23 |
|
Total wild prey |
66.67 |
59.46 |
54.17 |
57.58 |
59.68 |
|
Domestic prey |
|||||
|
Poultry (Gallus gallus domesticus) |
10 |
16.22 |
16.67 |
24.24 |
16.94 |
|
Total domestic prey |
10 |
16.22 |
16.67 |
24.24 |
16.94 |
|
Plants |
|||||
|
Melon (Cucumis melo) |
3.33 |
0 |
0 |
0 |
0.81 |
|
Chhoti bairi, fitni (Ziziphus oxyphylla) |
6.67 |
2.7 |
0 |
0 |
2.42 |
|
Water melon (Citrullus lanatus) |
3.33 |
0 |
0 |
0 |
0.81 |
|
Khabbal (Cynodon dactylon) |
0 |
2.7 |
0 |
3.03 |
1.61 |
|
Grass (Themeda anathera) |
0 |
0 |
8.33 |
9.09 |
4.03 |
|
Apple (Pyrus malus) |
0 |
2.7 |
4.17 |
0 |
1.61 |
|
Total plants |
13.33 |
8.1 |
12.5 |
12.12 |
11.29 |
|
Grits |
0 |
5.41 |
0 |
3.03 |
2.42 |
|
Anthropogenic |
10 |
10.81 |
16.67 |
3.03 |
9.68 |
Discussion
Diet composition of Indian grey mongoose consisted of mammals, birds, reptiles, amphibians, invertebrates, egg shells, and plant species. We also recorded grit and anthropogenic items. Frequency of occurrence of house mouse was high in the diet of Indian grey mongoose. Among birds, frequency of chicken/poultry was high. Four species/orders of invertebrates were recorded; frequency of occurrence of hymenopteran (ants, bees) was high and frequency of occurrence of Coleoptera (beetles) and snails was low. Our results are in line with other studies such as Roberts (1997) that the Indian mongoose fed on reptiles, birds, and amphibians. Prakash (1959) reported that in Rajasthan, Indian grey mongoose fed on grey partridges, rodents, invertebrates and lizards. Indian grey mongoose is known as an opportunistic hunter. It has been reported to feed on rodents, reptiles, invertebrates and birds, eggs of birds and fruits. We also recorded birds, egg shells, invertebrates, plants, reptiles and rodents in its diet. Such results have been reported in other studies from Sri Lanka that the Indian grey mongoose fed on the red jungle fowl Gallus gallus, its chicks, eggs, Indian blue peafowl Pavo cristatus, black and grey francolins Francolin francolinus and F. pondicerianus, small mammals and snakes (Santiapillai et al., 2000). It has also been reported to search for food under stones on the beach side in Hawaii (Santiapillai et al., 2000). It has been reported to feed on grasshoppers, centipedes, fish, frogs, scorpions and crabs (Whitfield, 1978). Hussain et al. (2017) reported that Indian grey mongoose fed on rodents, birds, insects, and plants in Pakistan.
During the current study, the diet of small Indian mongoose consisted of mammals, birds, invertebrates, reptiles, amphibians, and plants. We also recorded anthropogenic matter (plastic bags, and threads) and grit. Among mammals, frequency of occurrence of house rat was high. This could be due to the reason that small Indian mongoose lives around human habituations where availability of house rat might be high. Small Indian mongoose feeds on rodents such as house rat, and house mice when it lives around human settlements (Roberts, 1997). Only one species of domestic prey was recorded i.e., chicken/poultry. Species of invertebrates such as grasshoppers, ants, bees and beetles reported in this study is in line with the study conducted in India (Prakash, 1959). We recorded plant species such as Themeda anathera, Fitni beri, melon and water melon and plant matter in the diet of mongoose as reported earlier, that the species feeds on plants, beetles, scorpions, snakes, lizards, spiders and amphibians (Prakash, 1959). It feeds on birds and their eggs and nestlings (Roberts, 1997). Seaman and Randall (1962) reported that small Indian mongoose consumed small mammals, reptiles, amphibians, birds and plant matter. Some populations of mongoose are insectivorous and others may consume fruits during the same season (Seaman and Randall, 1962). Small Indian mongoose feeds on rodents and insects and plant matter (Siddiqui et al., 2004; Mahmood et al., 2011; Hussain et al., 2017).
Niche breadth of Indian grey mongoose was found broad 18 (0.72) during autumn season and narrow 7.8 (0.29) during winter season. Hussain et al. (2017) studied niche breadth and niche overlap between the two mongoose species in Potohar region, Pakistan. They reported that there was seasonal variation in niche breadth of Indian grey mongoose and it was high during summer season and narrow during winter season.
Dietary niche breadth of small Indian mongoose was broad during summer season 11.64 (0.59) and narrow during spring season 9.35 (0.46). Total niche breadth of small Indian mongoose was 12 (0.64). Hussain et al. (2017) reported niche breadth of small Indian mongoose being variable during different seasons and it was the highest (7.2) in summer and the lowest (6.1) in winter. The findings of the current study have also shown variation in niche breadth of small Indian mongoose during different seasons being high during summer season. But unlike Hussain et al. (2017), narrow niche breadth was recorded during spring season, which could be attributed to habitat variability and to variation in prey species availability during different seasons.
Niche overlap among small Indian mongoose and Indian grey mongoose was 0.89. There are not many previously published records to compare such findings except Hussain et al. (2017) who reported that there was high niche overlap (0.95) between two mongoose species in the Pothwar region Pakistan. Similar findings were documented during the current study.
Conclusions
The study concludes that niche of two mongoose species overlaps in the study area. The small Indian mongoose has a wider distribution as compared to Indian grey mongoose. The current research study provides baseline data on diet composition of the two-mongoose species from the study area. Because of high niche overlap both the mongoose species compete for resources; further studies on the ecology of both the species would be a vital step towards their conservation and management.
Acknowledgements
We are grateful to IDEA WILD and HEC Pakistan for their support during current research project. Without their support it would not have been possible for us to complete this research project. We are thankful to the anonymous reviewers for their constructive comments to improve this manuscript.
Statement of conflict of interest
The authors declare no conflict of interest.
References
Akhtar, N., Muhammad, R., Saeed, K., Khan, M.F., Shah, M., Zeb, J., Ahmad, S., Afridi, A.J. and Hussain, A., 2018. Distribution of wild mammalian fauna of Mahaban and Malka Valley District Buner. Pakistan J. Zool., 50: 775-777.
Akrim, F., Mahmood, T., Hussain, R. and Qasim, S., 2017. Distribution pattern, population estimation and threats to the Indian pangolin Manis crassicaudata (Mammalia: Pholidota: Manidae) in and around Pir Lasura National Park, Azad Jammu & Kashmir, Pakistan. J. Threat. Taxa, 9: 9920-9927. https://doi.org/10.11609/jott.2914.9.3.9920-9927
Akrim, F., Mahmood, T., Max, T., Nadeem, M.S., Qasim, S. and Andleeb, S., 2018a. Assessment of bias in morphological identification of carnivore scats confirmed with molecular scatology in north-eastern Himalayan region of Pakistan. PeerJ, 6: e5262. https://doi.org/10.7717/peerj.5262
Akrim, F., Mahmood, T., Nadeem, M.S., Andleeb, S. and Qasim, S., 2018b. Spatial distribution and dietary niche breadth of the leopard Panthera pardus (Carnivora: Felidae) in the northeastern Himalayan region of Pakistan. Turk. J. Zool., 42: 585-595. https://doi.org/10.3906/zoo-1803-2
Aragona, M. and Setz, E.Z., 2001. Diet of the maned wolf, Chrysocyon brachyurus (Mammalia: Canidae), during wet and dry seasons at Ibitipoca State Park, Brazil. J. Zool., 254: 131-136. https://doi.org/10.1017/S0952836901000620
Bang, P. and Dahlström, P., 1975. Huellas y señales de los animales de Europa. Omega, Barcelona, pp. 239.
Bujne, A.E., 2000. Pollen analysis of faeces as a method of demonstrating seasonal variations in the diet of Svalbard reindeer Rangifer tarandus platyrhynchus. Polar. Res., 19: 183-192. https://doi.org/10.1111/j.1751-8369.2000.tb00342.x
Camardella, A.R., Abreu, M.F. and Wang, E., 2000. Marsupials found in felid scats in southern Brazil, and a range extension of Monodelphis theresa. Mammalia, 64: 379-382.
Chinchilla, F.A., 1997. La dieta del jaguar (Panthera onca), el puma (Felis concolor) y el manigordo (Felis pardalis) (Carnivora: Felidae) en el Parque Nacional Corcovado, Costa Rica. Rev. Biol. Trop., 45: 1223-1229.
Colwell, R.K. and Futuyma, D.J., 1971. On the measurement of niche breadth and overlap. Ecology, 52: 567-576. https://doi.org/10.2307/1934144
Corbet, G.B. and Hill, J.E., 1992. The mammals of the Indo-Malayan region. Natural History Museum Publications, Oxford University Press, Oxford, UK, pp. 496.
Dalén, L., Gotherstrom, A. and Angerbjorn, A., 2004. Identifying species from pieces of faeces. Conserv. Genet., 5: 109-111. https://doi.org/10.1023/B:COGE.0000014060.54070.45
Ewer, R.F., 1973. The carnivores. Cornell University Press, Ithaca, NY, pp. 500.
Fedriani, J.M., Fuller, T.K., Sauvajot, R.M. and York, E.C., 2000. Competition and intraguild predation among three sympatric carnivores. Oecologia, 125: 258-270. https://doi.org/10.1007/s004420000448
Fragoso, J.M.V. and Huffman, J.M., 2000. Seed-dispersal and seedling recruitment patterns by last Neotropical megafaunal element in Amazon, the Tapir. J. trop. Ecol., 16: 369-385. https://doi.org/10.1017/S0266467400001462
Francis, C.M., 2008. A field guide to the mammals of South-East Asia. Princeton University Press, Princeton, New Jersey and Oxford, United Kingdom, pp. 392.
Gilchrist, J.S., Jennings, A.P., Veron, G. and Cavallini, P., 2009. Family Herpestidae. In: Handbook of the mammals of the world, Vol. 1: Carnivores (eds. D.E. Wilson and R.A. Mittermeier). Lynx Editions, Barcelona, Spain, pp. 656.
Harrison, D.L., 1968. The mammals of Arabia. Vol. II. Ernest Benn. London. pp. 193-381.
Hassinger, J., 1968. Introduction to the mammal survey of the 1965 street expedition to Afghanistan. Fieldiana Zool., 55: 1-18.
Hussain, R., Mahmood, T., Akrim, F., Fatima, H. and Nadeem, M.S., 2017. Human activity mediates reciprocal distribution and niche separation of two sympatric mongoose species in Pothwar Plateau, Pakistan. Turk. J. Zool., 41: 1045-1058. https://doi.org/10.3906/zoo-1606-34
Jackson, R. and Hunter, D.O., 1995. Snow leopard survey and conservation handbook, 3rd edn. International Snow Leopard Trust and U.S. National Biological Service, Seattle, pp. 120.
Jacomo, A.T.A., Silveira, L. and Diniz-Filho, J.A., 2004. Niche separation between the maned wolf (Chrysocyon brachyurus), the crab-eating fox (Dusicyon thous) and the hoary fox (Dusicyon vetulus) in central Brazil. J. Zool., 262: 99-106. https://doi.org/10.1017/S0952836903004473
Jenks, G.F. and Caspall, F.C., 1971. Error on choroplethic maps: definition, measurement, reduction. Annls. Assoc. Am. Geogr., 61: 217-244. https://doi.org/10.1111/j.1467-8306.1971.tb00779.x
Kauhala, K. and Auniola, M., 2001. Diet of raccoon dogs in summer in the Finnish archipelago. Ecography, 24: 151-156. https://doi.org/10.1034/j.1600-0587.2001.240205.x
Kohn, M.H., York, E.C., Kamradt, D.A., Haugt, G., Sauvajot, R.M. and Wayne, R.K., 1999. Estimating population size by genotyping faeces. Proc. R. Soc. B., 266: 657-663. https://doi.org/10.1098/rspb.1999.0686
Lavoie, G.K., 1971. Food habits: A technique for slide preparation. Technical Report, No. 69, Range Science Department, US International Biological Program, pp. 1-5.
Levins, R., 1968. Evolution in changing environments. Princeton University press, Princeton, NJ, pp. 120.
Mahmood, T., Hussain, I. and Nadeem, M.S., 2011. Population estimates, Habitat preference and the diet of small Indian mongoose (Herpestes javanicus) in Potohar Plateau. Pakistan J. Zool., 43: 103-111.
Mahmood, T., Niazi, F. and Nadeem, M.S., 2013. Diet composition of Asiatic jackal (Canis aureus) in Margallah hills national park, Islamabad, Pakistan. J. Anim. Pl. Sci., 23: 444-456.
Moore, T.D., Spence, L.E. and Dugnolle, C.E., 1974. Identification of the dorsal guard hairs of some mammals of Wyoming. In: Wyoming Game and Fish Department (Ed. W.G. Hopworth), Wyoming. pp. 177.
Nowak, R.M., 2005. Walker’s carnivores of the world. Johns Hopkins University Press, Baltimore, Maryland, pp. 313.
Oli, M.K., 1993. A key for the identification of the hair of mammals of a snow leopard (Panthera uncia) habitat in Nepal. J. Zool., 231: 71-93. https://doi.org/10.1111/j.1469-7998.1993.tb01924.x
Page, L.K., Swihart, R.K. and Kazacos, K.R., 2001. Seed preferences and foraging by granivores at raccoon latrines in the transmission dynamics of raccoon roundworm (Baylisascaris procyonis). Can. J. Zool., 79: 616-622. https://doi.org/10.1139/cjz-79-4-616
Pianka, E.R., 1973. The structure of lizard communities. Annu. Rev. Ecol. Syst., 4: 53-74. https://doi.org/10.1146/annurev.es.04.110173.000413
Prakash, I., 1959. Food of some Indian desert mammals. J. Biol. Sci., 2: 100-109.
Ray, J. and Sunquist, M., 2001. Trophic relations in a community of African rainforest carnivores. Oecologia, 127: 395-408. https://doi.org/10.1007/s004420000604
Roberts, T.J., 1997. The mammals of Pakistan. Oxford University Press, Karachi.
Santiapillai, C., de Silva, M. and Dissanayake, S., 2000. The status of mongooses (family: Herpestidae) in Ruhuna National Park, Sri Lanka. J. Bomb. Nat. Hist. Soc., 97: 208-214.
Santos, M.F.M. and Hartz, S.M., 1999. The food habits of Procyon cancrivorus (Carnivora, Procyonidae) in the Lami Biological Reserve, Porto Alegre, Southern Brazil. Mammalia, 63: 525-530.
Sanz, B., Turón, J.V. and Balmorí, A., 2007. Huellas y rastros de los mamíferos Ibéricos, 2nd ed. Ediciones Muskari, Zaragoza.
Seaman, G. and Randall, J., 1962. The mongoose as a predator in the Virgin Islands. J. Mammal., 43: 544-546. https://doi.org/10.2307/1376922
Seton, E.T., 1925. On the study of scatology. J. Mammal., 6: 47-49. https://doi.org/10.2307/1373469
Siddiqui, M.J.I., Rana, N. and Rana, S.A., 2004. Analysis of the scats of small Indian mongoose (Herpestes auropunctatus) with special reference to the insect fauna in croplands of Faisalabad, Pakistan. Entomology, 26: 95-99.
Walker, C., 1996. Signs of the wild. Struik Publish, Cape Town, pp. 215.
Webbon, C.C., Baker, P.J. and Harris, S., 2004. Faecal density counts for monitoring changes in red fox numbers in rural Britain. J. appl. Ecol., 41: 768-779. https://doi.org/10.1111/j.0021-8901.2004.00930.x
Wemmer, C., Kunz, T.H., Lundie-Jenkins, G. and McShea, W., 1996. Mammalian sign. In: Measuring and monitoring biological diversity - Standard methods for mammals (eds. D.E. Wilson, F.R. Cole, J.D. Nichols, R. Rudran and M.S. Foster). Smithsonian Institution Press, Washington, pp. 157-176.
Whitfield, P., 1978. The hunters. United States Department of Agriculture Circular, 118, Simon and Schuster, New York, pp. 1-4.
Williams, P.A., Karl, B.J., Bannister, P. and Lee, W.G., 2000. Small mammals as potencial seed disperses in New Zealand. Austral. Ecol., 25: 523-532. https://doi.org/10.1046/j.1442-9993.2000.01078.x
Wozencraft, W.C., 2005. Order carnivora. In: Mammal species of the world (eds. D.E. Wilson and D.M. Reeder). Smithsonian Institution Press, Washington and London.
To share on other social networks, click on any share button. What are these?










