Dissecting the Heat Stress Responses and Effects on Morphology of Tomato Varieties Employing Pre-transplant High Temperature Conditioning
Research Article
Dissecting the Heat Stress Responses and Effects on Morphology of Tomato Varieties Employing Pre-transplant High Temperature Conditioning
Gulzar Ullah* and Gohar Ayub
Department of Horticulture, Faculty of Crop Production Sciences, The University of Agriculture, Peshawar, Pakistan.
Abstract | An experiment was conducted to investigate the influence of heat pretreatment conditioning durations (control or no heat pretreatment,10 hrs,15 hrs and 20 hrs) on three tomato varieties (Roma, Rio Grande and Yaqui) sown at Horticulture Research Farm, Department of Horticulture, The University of Agriculture Peshawar during 2017 and 2018. Completely Randomized Design (CRD) in growth chamber before transplantation and Randomized Complete Block Design (RCBD) with two factors and three replications after transplanting to the field were used. Tomato seedlings at their 4-leaf stage were subjected to a 350C temperature stress for 10, 15 and 20 hrs as a pre-transplant hardening method. Both groups HP (heat pretreatment) and NHP (Non-heat pretreatment or Control) were then transplanted to the field. Results revealed that different heat pretreatment durations and varieties significantly affected the growth and yield of tomato. Regarding heat pretreatment durations, number of flower clusters plant-1 (16.06), fruit set percentage (68.33%), number of flowers cluster-1 (8.34), chlorophyll content (50.31 SPAD), leaf relative water content (75.34%), Putrescine concentration (193.95 nmol/g), spermidine concentration (154.59 nmol/g), spermine concentration (36.51 nmol/g), yield (20.14 t ha-1), early flowering (26.99 days) and lowest electrolyte leakage (48.87%) was observed in plants treated with 20 hours heat pretreatment duration as compared to other heat pretreatment durations and control. Concerning tomato varieties, number of flower clusters plant-1 (16.99), fruit set percentage (68.25%), number of flowers cluster-1 (7.91), chlorophyll content (48.06 SPAD), leaf relative water content (80.68%), Putrescine concentration (190.25 nmol/g), spermidine concentration (152.57 nmol/g), yield (21.00 t ha-1) and lowest electrolyte leakage (48.86%) was recorded for Rio Grande as compared to Roma and Yaqui. Also highest spermine concentration (35.72 nmol/g) and early flowering (26.72 days) resulted in Roma as compared to Rio Grande and Yaqui. The interaction effects of heat pretreatment duration and varieties were non-significant for most of the attributes studied except chlorophyll content and number of flower clusters plant-1. It can be concluded that heat pretreatment of 350C for 20 hours and Rio Grande variety resulted in enhanced growth, biochemical attributes and production of tomato in the agro-climatic conditions of Peshawar.
Received | August 09, 2021; Accepted | September 12, 2021; Published | October 01, 2021
*Correspondence | Gulzar Ullah, Department of Horticulture, Faculty of Crop Production Sciences, The University of Agriculture, Peshawar, Pakistan; Email: gulzarullah@aup.edu.pk
Citation | Ullah, G. and G. Ayub. 2021. Dissecting the heat stress responses and effects on morphology of tomato varieties employing pre-transplanthigh temperature conditioning. Sarhad Journal of Agriculture, 37(4): 1384-1402.
DOI | https://dx.doi.org/10.17582/journal.sja/2021/37.4.1384.1402
Keywords | Heat conditioning, Tomato, Temperature, Varieties, Yield
Introduction
Abiotic stresses including high or low light intensity, high or low temperature, salinity, metal toxicity and drought or excess moisture highly influence plant growth and productivity (Mittler and Blumwald, 2010; Cramer et al., 2011; Barnabas et al., 2008; Athar and Ashraf, 2009). High temperature is one of the most studied stresses that cause severe damage to the plant photosynthetic process leading to complete inhibition before other symptoms are detected (Berry and Bjoˆrkman, 1980). Plants can acclimate the changing climatic conditions driven by a rise in temperature which might sometimes result in a deleterious impact on plant physiology (Spicher et al., 2016). The influence on growth and survival directly depends on the duration as well as intensity of the heat stress. Exposure of the plants to a moderately high temperature for a longer duration can be as injurious as an extremely high temperature for a short duration (Georgieva, 1999).
For every specific plant, there exists an optimal temperature, at which the plant productivity is maximal. The most heat sensitive cell function is the chloroplast activity and a high temperature stress results in structural and functional damages. A high temperature stress leads to structural and functional changes in the chloroplast (Sato et al., 2006) and disturbs the hydraulic conductivity of roots and leaves (Morales et al., 2003). Furthermore, a high thermal stress results in reduced enzymatic activity (Ahmad et al., 2010), injuries to the cell membrane and alteration of cell differentiation, elongation and division (Potters et al., 2009; Rasheed, 2009; Smertenko et al., 1997). Eventually, due to decreased photosynthesis, plants have limited resource availability for reproduction in parental and gametophytic tissues leading to starvation (Sumesh et al., 2008; Young et al., 2004).
One of the key components of thermotolerance of a plant is protection against oxidative stress-induced by heat stress (Volkov et al., 2006; Suzuki et al., 2011; Wahid et al., 2007; Adachi et al., 2009). Although there are various hardening methods against temperature stress, high temperature preconditioning considerably decreases the heat induced damage in multiple crops (Colclough et al., 1990; Javanmardiet al., 2014). Studies have proved that tomato plants exhibited good osmotic adjustment as compared to non-conditioned or control plants (Morales et al., 2003). Plants adapt various strategies to tackle elevated temperature stress including short term acclimation and long term morphological and phenological modifications. Many plants adapt an escape mechanism by early maturation of the crop although associated with some yield loss (Adams et al., 2001). The mechanism of response to heat stress is different at different developmental stages and may also vary with respect to tissue type (Queitsch et al., 2000). Thermotolerance acquisition is an autonomous cellular mechanism which results from heat pretreatment of plants to a sublethal high temperature. This strategy protects the plant cells from a subsequent lethal high temperature stress (Vierling, 1991). This experiment aimed to investigate the influence of pre-transplant high temperature conditioning on the performance of tomato varieties in field conditions.
Materials and Methods
The experiment was carried out at Horticulture Research Farm, Department of Horticulture, The University of Agriculture, Peshawar during 2017 and 2018 to see the influence of Pre-transplant high temperature conditioning on the growth and development of tomato varieties under high temperature conditions.The experiment was laid out in a Completely randomized design (CRD) in a growth chamber before transplantation and Randomized Complete Block Design (RCBD) split plot with two factors (different heat pretreatment durations and tomato varieties) and three replications after transplanting in the field.
Seeds of all the three tomato varieties i.e. Roma, Rio Grande and Yaqui were obtained from a local market and were sown in January for raising nursery. Healthy seedlings at their 4- leaves stage were selected for the experiment and transferred to a growth chamber. After heat treatment, seedlings were transplanted to the field during March in the study period. The field was ploughed twice two weeks before the transplantation. Raised beds were prepared with a plant to plant distance of 60 cm and row to row distance of 75cm. The experimental field soil was silty loam in texture having a 7.7 pH. A recommended dose of NPK fertilizer in the form of Urea, Di ammonium phosphate (DAP), and Potassium Sulphate at the rate of 100, 90 and 60 kg ha-1 respectively were applied to the field. Potassium and phosphorus were applied to the soil before seedlings transplantation, while nitrogen fertilizer was given in two split doses. The first dose was given before transplantation, the second dose was provided after 30 days of seedling transplantation. The soil was well irrigated immediately after transplantation. While, later the irrigation process carried out according to the plant requirement. Manual weeding and hoeing regularly performed to avoid any weeds infestation.
Heat treatment procedure
There were two groups of seedlings; the first group was shifted to a growth chamber for pretreatment at 35 0C for 10, 15 and 20 hrs and then recovered at 25 0C for 2 hrs. The second group served as a control and was transplanted directly to fields without any heat pretreatment.
Parameters studied
Days to flowering
Data were calculated for the total number of days, when the first flower is formed in each randomly selected plant and the means were figured out.
Number of flowers cluster-1
Number of flowers per cluster was calculated for randomly selected plants and mean values were figured out.
Number of flower clusters plant-1
The data regarding number of flower clusters were recorded by counting the total number of flower clusters on each randomly selected plant and figuring out the mean values.
Electrolyte leakage
Total inorganic ions leaked out of the leaf was estimated by the method described by Ben Hamed et al. (2007). Twenty leaf discs were taken in a boiling test tube containing 10 mL of DDW, and electrical conductivity was measured (ECa). The tubes were heated at 45°C and 55 °C for 30 min in a water bath, and similarly electrical conductivity measured (ECb) each time. Later on, the contents were again boiled at 100 °C for 10 min, and electrical conductivity has been recorded again (ECc). The electrolyte leakage was calculated using the formula:
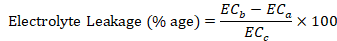
Chlorophyll content (SPAD)
Chlorophyll content was measured with the help of a chlorophyll meter (Model Number: TYS-A) by recoding the SPAD values of randomly selected leaves for each treatment per replication at the flowering stage.
Leaf relative water content
Fully expanded top most leaves were collected from the upper portion of the main shoot and fresh weight was elaborated. The leaves were immersed in distilled water in a Petri dish for two hrs. The leaves then removed, and the surface water was blottedoff to calculate the turgid weight. Samples had been dried in an oven at 70°C to constant weight.
The following formula used to determine the leaf relative water content (Turner, 1981):
LRWC (%) = [(F.W – D.W) / (T.W – D.W)] × 100 (1)
where F.W stands for Fresh weight; D.W for Dry weight and T.W represents Turgid weight.
Polyamines determination (Putrescine, Spermidine and Spermine)(nmol/g)
Freeze-dried tomato leaf material was extracted and dansylated as described by Torrigianiet al. (2012). with some modifications. Briefly, 50 mg of finely ground sample was homogenized in 800 µL of 5% ice-cold perchloric acid (PCA) using a hand-held homogenizer. The remaining procedure was exactly as described by Anwar et al. (2019). For PAs recovery and calibration curves, authentic PA standards (Sigma-Aldrich, USA) were used as control. PAs were integrated and quantified using Millennium 4.0 from Waters Corporation. PCA-soluble, PCA-soluble hydrolyzed with HCl, and PCA-insoluble after hydrolysis samples were quantified and designated as free, conjugated, and bound forms of each PA, respectively (Anwar et al., 2019).
Yield (t ha-1)
Yield per hectarewas determined by weighing tomato fruits of each treatment harvested from each subplot and then converting to tonnes using the formula:
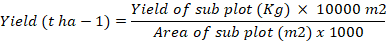
Results and Discussion
Days to flowering
The statistical analysis of the data concerning days to flowering indicated that heat pretreatment and varieties significantly affected the days to flowering of tomato (Table 1) while the interactions were non-significant. Mean values of the data showed that heat pretreatment of 20 hrs produced early flowering (26.99 days) in tomato, followed by 15 hrs HP (28.44 days), which was at par with that of 10 hrs HP (29.15 days). Late flowering occurred in the tomato plants treated with no HP (31.61 days). Concerning varieties, the highest days to flowering (31.57 days) was taken by Yaqui as compared to Roma plants which took the lowest days to flowering (26.72 days).
The seedling stage has a crucial role in tomato flowering and ultimately fruit production. This stage of plant growth greatly influences flowering to harvest time and its optimization can enhance the quality as well as production yield of tomato crop (Zhan et al., 2003). Flowering and fruit set are two key processes that are sensitive to high temperature and a basis for identifying heat tolerance in tomato (Berry and Uddin, 1988; Hanna and Hernandez, 1982). Thermotolerance is acquired from prior subjecting the plants to a sublethal high temperature pretreatment (Hong and Vierling, 2000). Early flowering with longer heat pretreatment duration might be related to the heat acclimated plants stress avoidance strategy to prevent the issues related to high temperature at the flowering stage (Peet et al., 1997). Heat preconditioning can reduce stress injury by stabilizing the membrane structure, regulating membrane functions, optimize the ion leakage and membrane permeability leading to seedlings tolerance to high temperature stress. Seedlings treated with a longer duration of heat pretreatment flowered earlier as compared to control plants. These results are in conformity with Javanmardi et al. (2009) who recorded an early flowering as well as maximum fruit set in heat preconditioned plants as compared to non-acclimated plants.
Number of flowers cluster-1 andnumber of flower clusters plant-1
Data regarding number of flowers cluster-1of tomato varieties is presented in Table 1. Statistical analysis of the data revealed that heat pretreatment and varieties significantly affected the number of flowers cluster-1of tomato plants while there was no significant difference regarding various interactions. The least number of flowers cluster-1 (5.61) were recorded in control tomato plants, while 20 hrs HP treated tomato plants resulted in highest number of flowers cluster-1(8.34) which was at par with those of 15 hrs (7.97). As far as varieties are concerned, Rio Grande plants produced highest number of flowers cluster-1(7.91)followed by Roma (7.48) while the lowest number of flowers per cluster(6.44)resulted in Yaqui plants.
Table 1: Effect of Pre-transplant high temperature conditioning on days to flowering, number of flowers cluster-1 and number of flower clusters plant-1of tomato varieties.
|
Heat Treatment (HT) (Hrs) |
Days to flowering |
Number of flowers cluster-1 |
Number of flower clusters plant-1 |
|
0 |
31.61 a |
5.61 c |
13.19 c |
|
10 |
29.15 b |
7.19 b |
15.31 b |
|
15 |
28.44 bc |
7.97 a |
15.72 ab |
|
20 |
26.99 c |
8.34 a |
16.06 a |
|
LSD at α 0.05 |
2.04 |
0.45 |
0.44 |
|
Varieties (V) |
|||
|
Roma |
26.72 c |
7.48 b |
15.31 b |
|
Rio Grande |
28.85 b |
7.91 a |
16.99 a |
|
Yaqui |
31.57 a |
6.44 c |
12.92 c |
|
LSD at α 0.05 |
3.49 |
0.13 |
0.61 |
|
Interactions (HT x V) |
NS |
NS |
*Fig 3.1 |
Means followed by similar letter(s) in column do not differ significantly from one another; NS = Non-significant and *, ** = Significant at 5 and 1% level of probability, respectively.
Number of flower clusters per plantof tomato was also significantly affected by heat pretreatment, varieties and interactions (Table 1). The number of flower clusters per plantof tomato were least (13.19), when treated with no HP as compared to the plants treated with 20 hrs HP that resulted in highest number of flower clusters per plant(16.06) followed by 15 hrs HP (15.72) and 10 hrs HP (15.31). Data regarding varieties revealed that the lowest number of flower clusters plant-1(12.92) was observed in Yaqui plants, while the highest number of flower clusters plant-1(16.99) was noted in Rio Grande plants. Interactive effects showed that number of flower clusters plant-1was improved with an increase in HP. However, the increase was maximum at 20 hrs HP in Rio Grande (Figure 1).
Based on the developmental stage, the mean daily temperature for optimum tomato growth and production is around 21 to 250C (Geisenberg and Stewart, 1986) and a rise in temperature of even a few degrees can adversely affect flowers and fruit set. Plant’s reproductive stages especially gametogenesis and fertilization are most crucial with respect to a rise in temperature. Moreover, meiosis occurs a few days before anthesis, which is a highly temperature sensitive phase of plant growth (Peet et al., 1997). Higher number of flowers in 15 hrs and 20 hrs heat pretreated plants was likely because of the seedlings being hardened by high temperature conditioning before transplantation. A decline in the number of flowers cluster-1 in control plants is similar to the results obtained by Javanmardi et al. (2009) who reported a limited reproductive growth in non heat pretreated plants as compared to heat pretreated plants. Furthermore, a longer duration of heat pretreatment of 20 hrs before transplantation enabled the plant to flower early, resulting in an escape from higher temperature stress around the flowering stage and ultimately higher number of flowers cluster-1 (Peet et al., 1997). An abrupt heat treatment (42–450C) also enables the plants to acclimate high temperature stress during the flowering stage (Larkindale and Vierling, 2008; Suzuki et al., 2008). Qin et al. (2008) applied high temperature treatment of 36–380C to Arabidopsis thaliana plants for a short period as a hardening method and then subjected the plants to increased durations of 450C temperature stress to acquire thermotolerance. Another research investigated the effect of a gradual rise in temperature to achieve acclimation in Arabidopsis thaliana seedlings to heat stress of 450C. A massive build-up of heat shock proteins as well as reactive oxygen species and enzymes was observed during the treatment (Larkindale and Vierling, 2008).
Fruit set percentage (%)
Data concerning the fruit set percentage of the tomato crop in response to heat pretreatment and varieties is shown in Table 2. Fruit set percentage was significantly affected by different heat pretreatment levels and varieties while the interaction effect of all the treatments was non-significant. The fruit set percentage increased from (60.70%) in control plants to (68.33%) with 20 hrs HP application, followed by (64.64%) with 15 hrs HP treatment. The highest fruit set percentage (68.25%) was recorded in Rio Grande plants, followed by (64.33%) in Roma, while the lowest fruit set percentage (58.36%) resulted in Yaqui plants.
Plants parts that are most sensitive to high temperature stress are floral and fruiting organs (Wahid et al., 2007). Acquired thermotolerance at the anthesis stage can play a key role in determining the final yield. Higher fruit set percentage in heat pretreated plants for a longer duration might be due to good osmotic adjustment, enabling the plant to maintain leaf pressure potential at an optimum stage during the heat stress conditions leading to normal growth and development. Similar results were also produced by Morales et al. (2003) who studied the influence of high temperature conditioning of 30°C and 35°C as preconditioning temperatures and concluded that preconditioned plants had higher pressure potential as well as stomatal conductance as compared to those of non-conditioned plants. Osmolyte production is also known to enhance protein stability and membrane integrity under heat stress conditions (Mirzaei et al., 2012; Sung et al., 2003). Javanmardi et al. (2014) investigated the influence of high temperature pretreatment in tomato and observed a higher fruit set and early first flower initiation in heat pretreated plants in comparison with control plants. Heat pretreatment also enables the plant to cope with high temperature stress due to its enhanced membrane integrity, higher levels of proline and carbohydrate content, antioxidant enzyme activities and reduced chlorophyll degradation (Ding et al., 2016).
Significant influence of varieties was noted regarding fruit set percentage. This was in accordance with (Zhou, 2017) who investigated the physiological response of different tomato genotypes to heat stress during seedling as well as anthesis stage and reported a significant influence of cultivars on fruit set of tomato. The heat tolerant genotype continued normal truss formation under high temperature stress indicating normal floral organ growth and development. Also the heat tolerant cultivars maintained a higher level of carbohydrates assimilation and nutrients uptake
Table 2: Effect of Pre-transplant high temperature conditioning on fruit set percentage (%), electrolyte Leakage (%) chlorophyll content (SPAD) and leaf relative water content (%) of tomato varieties.
|
Heat Treatment (HT) (Hrs) |
Fruit set percentage (%) |
Electrolyte Leakage (%) |
Chlorophyll content (SPAD) |
Leaf relative water content (%) |
|
0 |
60.70 c |
57.77 a |
41.09 c |
62.19 c |
|
10 |
60.88 c |
55.87 b |
43.76 b |
70.22 b |
|
15 |
64.68 b |
51.43 c |
45.59 b |
72.51 ab |
|
20 |
68.33 a |
48.87 d |
50.31 a |
75.34 a |
|
LSD at α 0.05 |
2.71 |
1.78 |
2.13 |
2.89 |
|
Varieties (V) |
||||
|
Roma |
64.33 b |
52.36 b |
45.28 b |
67.05 b |
|
Rio Grande |
68.25 a |
48.86 c |
48.06 a |
80.68 a |
|
Yaqui |
58.36 c |
59.24 a |
42.23 c |
62.48 c |
|
LSD at α 0.05 |
2.14 |
3.06 |
3.28 |
3.34 |
|
Interactions (HT x V) |
NS |
NS |
*Fig 3.2 |
NS |
Means followed by similar letter(s) in column do not differ significantly from one another; NS = Non-significant and *, ** = Significant at 5 and 1% level of probability, respectively.
(Kumari et al., 2013; Vignjevic et al., 2015) as well as cell wall and vascular invertases activities (Li et al., 2012) under high temperature stress conditions ultimately leading to better fruit set in tomato. These results were also in conformity with (Abdul Baki, 1991), who concluded that tolerant cultivars resulted in more flowers, optimum matured fruits, highest fruit set percentage and highest yield as compared to heat sensitive cultivars both in a greenhouse as well as under field conditions. Severe alterations in photosynthetic rate are induced by heat stress reducing the plant’s ability to mitigate the damaging effect of high temperature. Reduced antioxidant capacity and increased dark respiration in heat sensitive cultivar might be the reason for poor performance regarding fruit set in the field (Camejo, 2006).
Electrolyte Leakage (%)
It is clear from Table 2 for electrolyte leakage that there was a significant difference among different heat pretreatment and varieties. The interaction effects for all the treatments remained non-significant. Heat pretreatment of tomato seedlings for a 20 hrs duration resulted in minimum electrolyte leakage (48.87%), followed by (51.43%) with heat pretreatment for 15 hrs duration while maximum electrolyte leakage (57.77%) was recorded in control plants. The mean data regarding varieties showed that minimum electrolyte leakage (48.86%) was noted for Rio Grande plants, while the maximum electrolyte leakage (59.24%) was observed in Yaqui plants.
The temperature as well as duration of heat stress pretreatments, determines the growth and development of the plant under heat stress conditions and the response of the plants vary between growth stages and tissue type. Maintenance of homeostasis and repairing of heat sensitive components provides an insight in the ability of the plant to withstand or acclimate high temperature stress. Higher percentage of electrolyte leakage is an indicator of increased oxidative processes, reduced heat shock proteins production and alterations in membrane permeability resulting in oxidative damage and heat injury to the plant system (Vierling, 1991; Kotak et al., 2007). Reduced membrane integrity as a result of higher electrolyte leakage percentage, lower the ability of the plants to retain solutes as well as water in heat stress conditions (Allakhverdiev et al., 2008; Wahid and Shabbir, 2005). To prevent higher electrolyte leakage due to heat stress and to regulate metabolism, plant increases saturated and monounsaturated fatty acids content (Zhang et al., 2005b) which ultimately leads to membrane stability and enhanced heat tolerance (Larkindale and Huang, 2004). Several studies in tomato report that heat treatment induces secondary metabolites accumulation which protects the plant against oxidative damage (Rivero et al., 2001). Heat pretreatment also results in stabilized membrane structure and functions, reduce electrolyte leakage as well as membrane permeability. These results are also in accordance with Javanmardi et al. (2014) who reported reduced electrolyte leakage in heat pretreated seedlings as compared to the non-heat pretreated seedlings in pepper and tomato.
Varieties were also significantly different regarding electrolyte leakage percentage. Reduced electrolyte leakage is a characteristic feature of heat tolerant variety when exposed to high temperature stress along with higher photosynthetic activity and heat avoidance (Nagarajan et al., 2010; Scafaro et al., 2010). Certain genotypes are more tolerant to high temperature and their response to heat stress directly depend on genotypic parameters (Challinor et al., 2007; Prasad et al., 2006). However, (Camejo et al., 2006) also reported an increase in electrolyte leakage in heat sensitive cultivar relating it to alterations in membrane permeability and reduced solute and water retention ability due to high temperature stress. A heat tolerant cultivar Nagcarlang was not influenced by the heat stress and retained its membrane permeability showing the maintenance of its various functioning processes. A higher level of electrolyte leakage is an indicator of reduced functioning of mitochondrial and photosynthetic systems in plant cells (Shanahan et al., 1990; Ristic et al., 1996).
Chlorophyll content (SPAD)
Table 2 shows that heat pretreatment and varieties significantly affected the chlorophyll content of tomato plants. The interactions between heat pretreatment and varieties were also significant. Concerning HP, the highest chlorophyll content (50.31 SPAD) was recorded in plants subjected to 20 hrs heat pretreatment duration, followed by (45.59 SPAD) with 15 hrs heat pretreatment duration, whereas the lowest chlorophyll content (41.09 SPAD) resulted in plants treated with no heat pretreatment duration (Control). Rio Grande plants resulted in the highest chlorophyll content (48.06 SPAD) as compared to those of Yaqui plants that recorded the lowest chlorophyll content (42.23 SPAD). The interaction between heat pretreatment and varieties showed that the chlorophyll content increase with an increase in heat pretreatment duration for all varieties, however heat pretreatment of 20 hrs duration in Rio Grande plants resulted in the highest chlorophyll content (Figure 2).
High temperature stress significantly influences various photosynthetic parameters like chlorophyll a/b levels and chlorophyll/carotenoid ratio and tremendously disturbs the root and leaf water hydraulic conductivity (Wahid, 2007; Morales et al., 2003). Chloroplast is the major source of reactive oxygen species (ROS) in the plant cell. They produce superoxide radicals and singlet oxygen from the harvested light energy (Salin, 1988). Under various stress conditions like drought, heat and herbicides, photoactivated chlorophyll also excite oxygen to singlet form when the harvested light energy is not used in the electron transport system (Foyer, 2002). Several research studies have confirmed that the association of chlorophyll content with plant heat stress, the results indicates that cultivars having a high greenness level are less prone to high temperature stress (Lopes and Reynolds, 2012; Reynolds et al., 2012; Rossi et al., 2017; Wang et al., 2017). Furthermore, various reports are indicating a devastating influence of high temperature stress on chlorophyll biosynthesis in different plant species (Feierabend, 1977; Tewari and Tripathy, 1998; Takahashi et al., 2008).
Chlorophyll content also gives an idea of the nutrient absorption as well as photosynthetic assimilation ability of a plant. An increase in chlorophyll content was observed with increasing heat pretreatment duration. The highest chlorophyll content resulted in plants treated with 20 hrs heat pretreatment. Further, Javanmardi et al. (2014) investigated the influence of heat preconditioning on chlorophyll content of tomato and pepper leaves and reported that tomato and pepper plants produced a higher chlorophyll content when preconditioned with heat treatment as compared to the non-acclimated plants. An improved chloroplast structure under high temperature stress is also reported to be a result of heat acclimation (Xu et al., 2006). Besides, Morales et al. (2003) studied the effect of high temperature preconditioning and thermal shock on tomato cultivar Amalia and reported a significant influence of high temperature pretreatment on chlorophyll content, stomatal conductance as well as net photosynthetic rate. Heat acclimated plants had a higher degree of thermo-stability, lower chloroplast damage and lipid peroxidation as compared to the non-heat acclimated plants when subjected to a high temperature stress (Xu et al., 2006). The findings are also in conformity with Ibrahim and El-Muqadam (2019) who also obtained similar results and recorded a higher chlorophyll in plants preconditioned with a high temperature hardening treatment. Slower heat treatment results in serious plant injuries including loss of membrane integrity as well as enzymes inactivation in the chloroplast (Howarth, 2005). Similar, findings were also obtained by Dutta et al. (2009) and Reda and Mandura (2011) who recorded enzymes destruction that regulates chlorophyll synthesis under high temperature stress.
Varieties were also significantly different from one another regarding the chlorophyll content of tomato. Moreover, Wahid and Ghazanfar (2006) observed similar results and concluded that tolerant tomato genotypes produced a higher chlorophyll a:b ratio and a lower chlorophyll: carotenoids ratio when exposed to a higher than optimum temperature stress. These parameters are also used as a tool for indicating thermotolerance and physiological status in tomato plants. In addition, Camejo (2005) investigated the influence of high temperature on photosynthetic activity of two tomato cultivars and recorded an increase in chlorophyll a/b ratio and decrease in chlorophyll/carotenoid ratio in heat stressed Nagcarlang tomato plants. Thermotolerance development in plants also relates to the amount of chloroplast protein synthesis elongation factor under high temperature stress (Moriarty et al., 2002). Heat shock proteins in chloroplast protect the plant from the adverse effects of high temperature stress ad play a key role in electron transport (Barua et al., 2003). Accumulation of chlorophyll has also been correlated with thermotolerance in various crop species (Selvaraj et al. 2011).
Zhou et al. (2015) studied the physiological response of heat stress in different tomato genotypes with different types of heat susceptibility and reported that cultivar ‘LA1994’ resulting in an altered photosynthetic rate and chlorophyll content as compared to control plants when exposed to a 36/28 °C temperature stress. In support of our findings, Gosavi et al. (2014) and Zhou et al. (2017) also recorded an increase in total chlorophyll in tolerant genotypes as compared to the susceptible ones.
Leaf relative water content (%)
The leaf relative water content was significantly affected by heat pretreatment and varieties while their interaction was not significant (Table 2). Heat pretreatment of tomato seedlings for a 20 hrs duration resulted in the highest leaf relative water content (75.34%), followed by (72.51%) with heat pretreatment for 15 hrs duration while the lowest leaf relative water content (62.19 %) was recorded in control plants. The highest leaf relative water content (80.68%) was recorded in Rio Grande plants, followed by (67.05%) in Roma, while the lowest leaf relative water content (62.48%) resulted in Yaqui plants.
The best way to judge plant water status of cellular water deficit under various stressful conditions is the relative water content (Sekmen et al. 2014). A decrease in leaf water content causes leaf desiccation, which initially causes the stomata closure followed by stromal photosynthetic reactions ultimately results in photosynthetic inhibition (Bertolli et al. 2012; Zivcak et al. 2013). Higher leaf temperature is correlated with an increase in temperature either directly by having some damaging effects in plant tissues or indirectly leads to an increase in plant water deficit. Both leaf relative water content and turgidity can be used as indicators of growth and development under high temperature stress, an increase in temperature triggers evaporative demands and ultimately results in higher transpiration rates as well as reduced water potential (Hall, 2000). High temperature is also considered as the most influential factor that causes a higher plant evaporation demand ultimately contributing towards water deficiency (Karim et al. 1997; Simoes-Araujo et al., 2003). Turgidity maintenance is an important factor in optimum plant growth as well as implementing normal cell activities (Farouk, 2011). It is also worth mentioning that decreased leaf relative water content and turgidity loss occur with an increase in transpiration due to high temperature (Cansev, 2012; Turkes, 2003; Yamasaki and Dillenburg, 1999). Studies have confirmed that a decline in relative water content plays a key role in decreased growth in response to osmotic stress in plants (Alexieva et al., 2001). A decrease in leaf relative water content in control plants might be due to not being hardened by heat pretreatment to cope with the heat stress of field conditions after transplanting. Better water retention in heat hardened tomato plants may also be associated with better osmoregulation as well as healthy vegetative growth in the field (Hasthanasombut et al., 2011). These results are in conformity with other researchers (Khalil and Moursy 1983) who reported promoted growth of various plants due to heat hardening.
Zhou et al. (2017) subjected three tomato cultivars to heat and drought stress and recorded a significant decrease in relative water content of all studied cultivars as compared to those of control plants. This also confirms the similar sensitive behavior of all three cultivars to the applied stress. Heat tolerant cultivars have normal growth and development under heat stress conditions because they have improved photosynthesis, water and nutrient use efficiency, membrane stability and assimilate partitioning as compared to heat sensitive cultivars (Camejo et al., 2005; Ahn and Zimmerman, 2006; Momcilovic and Ristic, 2007).
Polyamines (Putrescine, Spermidine and Spermine) (nmol/g)
Data for Putrescine is indicated in Table 3 which elaborates that heat pretreatment and varieties significantly affected Putrescine concentration. The interaction effect of heat pretreatment and varieties didnot show a significant variation in response to Putrescine concentration in tomato plants. The mean data regarding Putrescine concentration revealed that heat pretreatment of tomato plants for 20 hrs duration produced the highest Putrescine concentration (193.95 nmol/g), followed by (185.55 nmol/g), while the lowest Putrescine concentration (173.19nmol/g) was noted in tomato plants applied with no heat pretreatment (Control). Regarding varieties, The Putrescine concentration was highest (190.25 nmol/g) in Rio Grande, followed by (181.68 nmol/g) in Roma while the lowest Putrescine concentration (176.92 nmol/g) was observed in Yaqui plants.
The mean data in Table 3 showed that spermidine was significantly affected by various heat pretreatment durations and varieties. Interactions have no significant effect on the spermidine concentration of tomato plants. Regarding heat pretreatment duration, the highest spermidine concentration (154.59 nmol/g) was recorded in plants subjected to 20 hrs heat pretreatment duration, followed by (152.31 nmol/g) with 15 hrs heat pretreatment duration, whereas the lowest spermidine concentration (138.60 nmol/g) resulted in plants treated with no heat pretreatment duration (Control). The mean data regarding varieties showed that the lowest spermidine concentration (136.58 nmol/g) was noted for Rio Grande plants while the highest spermidine concentration (152.57 nmol/g) was observed in Yaqui plants.
Table 3: Effect of Pre-transplant high temperature conditioning on spermidine (nmol/g), spermine (nmol/g) and yield (t ha-1) of tomato varieties.
|
Heat Treatment (HT) (Hrs) |
Putrescine (nmol/g) |
Spermidine (nmol/g) |
Spermine (nmol/g) |
Yield (t ha-1) |
|
0 |
173.19 c |
138.60 b |
27.90 c |
18.18 c |
|
10 |
179.10 c |
139.33 b |
30.36 bc |
18.31 c |
|
15 |
185.55 b |
152.31 a |
32.04 b |
19.21 b |
|
20 |
193.95 a |
154.59 a |
36.51 a |
20.14 a |
|
LSD at α 0.05 |
2.75 |
3.72 |
3.02 |
0.47 |
|
Varieties (V) |
||||
|
Roma |
181.68 b |
149.47 b |
35.72 a |
18.86 b |
|
Rio Grande |
190.25 a |
152.57 a |
33.21 b |
21.00 a |
|
Yaqui |
176.92 c |
136.58 c |
26.18 c |
17.03 c |
|
LSD at α 0.05 |
5.15 |
3.32 |
3.91 |
0.37 |
|
Interactions (HT x V) |
NS |
NS |
NS |
NS |
Means followed by similar letter(s) in column do not differ significantly from one another; NS = Non-significant and *, ** = Significant at 5 and 1% level of probability, respectively.
Data regarding spermine concentration in Table 3 revealed that spermine concentration was significantly influenced by heat pretreatment durations and varieties. The interaction between heat pretreatment durations and varieties was not significant. Heat pretreatment of tomato seedlings for a 20 hrs duration resulted in increased spermine concentration (36.51 nmol/g), followed by (32.04 nmol/g) with heat pretreatment for 15 hrs duration while a decrease in spermine concentration (27.90 nmol/g) was recorded in control plants. The studied tomato varieties were also significantly different from one another regarding spermine concentration. Roma plants resulted in the highest chlorophyll content (35.72 nmol/g), followed by Rio Grande (32.21 nmol/g), as compared to those of Yaqui plants that recorded the lowest chlorophyll content (26.18 nmol/g).
Polyamines, mainly putrescine (Put), spermidine (Spd), and spermine (Spm) are ubiquitous, low molecular weight compounds that are widely distributed in all living organisms (Hussain et al., 2011). For instance, spermidine and spermine synthesis occur in shoot apical meristem in the case of tobacco whereas putrescine is produced in roots (Moschou et al., 2008). In most cases, polyamines in plants tend to work in adaptive responses to various abiotic stresses and their levels are severely influenced by different stress conditions. Liu et al. (2007) has investigated the variations in levels of polyamines by subjecting plants to a range of abiotic stresses like high salinity, high and low temperatures, drought, nutrient deficiency and recorded a significant change in polyamine levels. This change is higher in case of putrescine while spermidine and spermine show a minor increase in case of apple callus when treated with salt (Liu et al., 2006). These results also suggest that changes in polyamine levels are decided by various factors including plant species under investigation, type and condition of stress, stress tolerance capacity of the plant and its physiological status. Increased accumulation of polyamines maintains membrane stability under stress conditions by regulating antioxidant activities and binding strongly with negative charges in proteins and phospholipids (Asthir et al., 2004; Liu et al., 2007; Tiburcio et al., 2014). Polyamines also enhance various growth and developmental processes in plants including stimulation, support and development of flower buds, fruit set, fruit ripening, cell division and response to abiotic stresses (Bouchereau et al., 1999; Groppa and Benavides 2008).
Polyamines promote the photosynthetic activity of chloroplast and prevent lipids peroxidation (Drolet et al., 1986; Floryszak et al., 1992a). Putrescine plays a key role in mitigating harmful effects of stress because of its higher content as compared to spermidine and spermine leading to reduced oxidative damage (Bouchereau et al., 1999). Heat pretreatment hardened tomato plants and produced the optimum amount of polyamines necessary for better growth and development. Similar results were also observed by Goyal and Asthir (2010) who recorded promoted diamine oxidase and polyamine oxidase activities along with an increase in spermidine and spermine content in wheat under high temperature stress conditions. Similar results were also reported in multiple studies regarding an increase in polyamines in different crops and conditions like chickpea and soybean (Nayyar et al., 2005) under drought stress while in Arabidopsis (Sagor et al., 2013), tobacco (Cvikrová et al., 2012), and alfalfa (Königshofer and Lechner 2002) under heat stress conditions. Various research investigations have shown that polyamines role in stress tolerance is mainly by reactive oxygen species ROS homeostasis regulations (Liu et al., 2015). Exogenous application of polyamines as well as over expression of various polyamine biosynthetic genes enhanced plant ability to tolerate various abiotic stresses (Farooq et al., 2009, Wang et al., 2011a; 2011b; Fu et al., 2014). Various research studies have shown that stress conditions lead to polyamines accumulation but there are some reports of decrease or minimal polyamine alteration when experiments were performed under different conditions (Liu et al., 2006; 2007; 2008).
Considering cultivars, multiple research studies revealed that tolerant cultivars synthesize more spermidine and spermine as compared to sensitive cultivars which tend to produce more putrescine while having the same stress conditions (Krishnamurthy and Bhagwat, 1989; Santa-Cruz et al., 1998; Liu et al., 2004). Overall, heat tolerant cultivars have the ability to accumulate a higher quantity of polyamines as compared to heat sensitive cultivars (Hatmi et al., 2015), Enhanced levels of polyamines confer increased tolerance to different plants in multiple stress conditions (Duan et al., 2008).
Yield (t ha-1)
The yield of tomato cropwas significantly affected by heat pretreatment durations and varieties. However, the interaction between heat pretreatment durations and varieties was not significant (Table 3). Yieldincreased with an increase in heat pretreatment duration. The highest yield (20.14 t ha-1) resulted in tomato plants applied with 20 hrs heat pretreatment duration, followed by (19.21 t ha-1) with 15 hrs heat pretreatment duration, whereas the lowest yield (18.18 t ha-1) was observed in tomato plants subjected to no heat pretreatment (Control). Regarding various varieties studied during the experiment, the yield was highest (21.00 t ha-1) in Rio Grande plants, followed by (18.86 t ha-1) in Roma while the lowest yield (17.03 t ha-1) was noted in Yaqui plants.
High temperature stress directly as well as indirectly influence the overall yield of tomato. Direct damages of extreme temperature include increased membrane lipid fluidity, proteins denaturation and inhibition as well as enzyme inactivation of mitochondria and chloroplast (Howarth, 2005). A decline in fruit quality and yield of tomato is reported because of poor performance of crop at various developmental stages ranging from vegetative particularly leaves (Camejo et al., 2006; Shanmugam et al., 2013; Sharma et al., 2014) to reproductive growth (Rudich et al., 1977; Abdul-Baki 1991; Firon et al., 2006) under heat stress conditions. However, the reproductive stage is termed to be most sensitive stage to heat stress in various crops like wheat (Farooq et al., 2011; Shanmugam et al., 2013), tomato (Abdul Baki 1991; Sato et al., 2000) and cotton (Kakani et al., 2005; Snider et al., 2009). High temperature also has a significant damaging effect on at cellular, sub-cellular as well as tissue level of plant structures, ultimately leads to lowered crop yield and development (Wahid et al., 2007). Even at certain extreme high temperature and drought conditions, about 50% crop yield loss has also been reported (Wang et al., 2003). Other researchers recorded significant damage to intermolecular interactions needed for optimum growth and development as a result of high temperature stress (Bita and Gerats, 2013).
Considerable research work has been done to evaluate the influence of heat stress caused by elevated temperatures and its subsequent biochemical, physiological, anatomical, morphological and genetic response in decreasing crop yield (Snider et al., 2010; Camejo et al., 2005; Chen et al., 2012; Vasseur et al., 2011; Min et al., 2014). Heat pretreatment of tomato seedlings enhances yield performance in the field after transplanting. Our results show that an increased duration of heat pretreatment given to tomato seedlings act as a hardening tool to significantly increased yield of tomato crop. The optimum yield of heat pretreated plants was also associated with the ability of hardened plants to maintain high nutrient uptake and carbohydrates assimilation during heat stress (Kumari et al., 2013; Vignjevic et al., 2015). These findings are also in accordance with Yarwood (1961) who reported that leaves exposed to high temperature stress for a specific duration can harden it to bear increased temperature stress than non-treated leaves.
Significant variation yield of tomato crop is also observed regarding different cultivars studied. The influence of heat stress and the response of plants vary between genotypes and stages of development (Wahid et al., 2007). For instance, carbohydrate metabolism and photosynthesis at the leaves stage varies between cultivars and is used as an indicator of heat susceptibility in plants (Camejo et al., 2006; Sharma et al., 2014).
Conclusions and Recommendations
From the results of the experiment, it was concluded that heat pretreatment of 350C for 20 hrs recorded the highest reproductive as well as biochemical attributes.Therefore, it is recommended that as a hardening method for tomato transplants to cope with the high temperature stress in field conditions. The Rio Grande variety performed better than other studied varieties, resulting inincreased reproductive growth and recommended for cultivation in the agro-climatic conditions of Peshawar, Khyber Pakhtunkhwa-Pakistan. It was also revealed from the results of the experiment that Roma resulted in the earliest flowering and produced the highest spermine content in all treated plants as well as control.
Acknowledgements
I am thankful to HEC, Pakistan for providing me financial assistance under “Indigenous 5000 PhD Fellowship Program” through which I have completed my PhD degree program.
Novelty Statement
For the first time, heat conditioning experiments were conducted on the local tomato cultivars of Peshawar, addressing the issues being faced by the farmers of the tomato growers in the study area.
Author’s Contribution
Gulzar Ullah: Principal Author and PhD scholar, designed and conducted research, data collection, analysis and writing of this manuscript.
Gohar Ayub: Major Supervisor, who provided technical guidelines in the whole PhD study and research.
Conflict of interest
The author’s declare no conflict of interest
References
Abdul-Baki, A.A. 1991. Tolerance of tomato cultivars and selected germplasm to heat stress. J. Am. Soc. Hort. Sci., 116 (6):1113 – 1116. https://doi.org/10.21273/JASHS.116.6.1113
Adachi, M., Y. Liu, K. Fujii, S.K. Calderwood, A. Nakai, K. Imai and Y. Shinomura. 2009. Oxidative stress impairs the heat stress response and delays unfolded protein recovery. PLoS One., 4:(11): e7719. https://doi.org/10.1371/journal.pone.0007719
Adams, S.R., K.E. Cockshull and C.R.J. Cave. 2001. Effect of temperature on the growth and development of tomato fruits. Ann. Bot., 88 (05): 869–877. https://doi.org/10.1006/anbo.2001.1524
Ahmad, A., H. Diwan and Y.P. Abrol. 2010. Global climate change, stress and plant productivity. Abiotic Stress Adaptation in Plants. 503-521. https://doi.org/10.1007/978-90-481-3112-9_23
Ahn, Y.J. and J.L. Zimmerman. 2006. Introduction of the carrot HSP17.7 into potato (Solanum tuberosum L.) enhances cellular membrane stability and tuberization in vitro. Plant, Cell Environ., 29(1): 95-104. https://doi.org/10.1111/j.1365-3040.2005.01403.x
Alexieva, V., I. Sergiev, S. Mapelli and E. Karanov. 2001. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant, Cell Environ. ,24(12):1337-44. https://doi.org/10.1046/j.1365-3040.2001.00778.x
Allakhverdiev, S.I., V.D. Kreslavski, V. V. Klimov, D.A. Los, R. Carpentier and P. Mohanty. 2008. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res., 98(1-3):541-550. https://doi.org/10.1007/s11120-008-9331-0
Anwar, R., S. Fatima, A.K. Mattoo and A.K. Handa. 2019. Fruit architecture in polyamine-rich tomato germplasm is determined via a medley of cell cycle, cell expansion, and fruit shape genes. Plants, 8(10):387. https://doi.org/10.3390/plants8100387
Asthir, B., W. Spoor and C.M. Duffus. 2004. Involvement of polyamines, diamine oxidase and polyamine oxidase in resistance of barley to Blumeriagraminis f. sp. hordei. Euphytica, 136(3): 307-312. https://doi.org/10.1023/B:EUPH.0000032730.48474.b1
Barua, D., C.A. Downs and S.A. Heckathorn. 2003. Variation in chloroplast small heat-shock protein function is a major determinant of variation in thermotolerance of photosynthetic electron transport among ecotypes of Chenopodium album. Funct. Plant Biol., 30(10):1071-1079. https://doi.org/10.1071/FP03106
Ben Hamed, K., A. Castagna, E. Salem, A. Ranieri and C. Abdelly. 2007. Sea fennel (Crithmummaritimum L.) under salinity conditions: A comparison of leaf and root antioxidant responses. Plant Growth Regul., 53(3):185-194. https://doi.org/10.1007/s10725-007-9217-8
Berry, J. and O. Bjorkman. 1980. Photosynthesis response and adaptation to temperature in higher plants. Annu. Rev. Plant. Physiol., 31(1): 491 – 543. https://doi.org/10.1146/annurev.pp.31.060180.002423
Berry, S. and M. Uddin. 1988. Effect of high temperature on fruit set in tomato cultivars and selected germplasm. Hort. Sci., 23(3): 606-608.
Bertolli, S.C., G.L. Rapchan and G.M. Souza. 2012. Photosynthetic limitations caused by different rates of water-deficit induction in Glycine max and Vigna unguiculata. Photosynthetica, 50(3):329-36. https://doi.org/10.1007/s11099-012-0036-4
Bita, C.E. and T. Gerats. 2013. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci., 4: 273. https://doi.org/10.3389/fpls.2013.00273
Bouchereau, A., A. Aziz, F. Larher and J. Martin-Tanguy. 1999. Polyamines and environmental challenges: Recent development. Plant Sci., 140(2): 103-125. https://doi.org/10.1016/S0168-9452(98)00218-0
Camejo, D., A. Jiménez, J.J. Alarcón, W. Torres, J.M. Gómez and F. Sevilla. 2006. Changes in photosynthetic parameters and antioxidant activities following heat-shock treatment in tomato plants. Funct. Plant Biol., 33(2):177-87. https://doi.org/10.1071/FP05067
Camejo, D., P. Rodríguez, M.A. Morales, J.M. Dell’Amico, A. Torrecillas and J.J. Alarcón. 2005. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol., 162(3):281-9. https://doi.org/10.1016/j.jplph.2004.07.014
Cansev, A. 2012. Physiological effects of high temperature treatments on leaves of olive cv. gemlik. Plant Arch., 12(1):521-5.
Challinor, A.J., T.R. Wheeler, P.Q. Craufurd, C.A.T. Ferro and D.B. Stephenson. 2007. Adaptation of crops to climate change through genotypic responses to mean and extreme temperatures. Agric. Ecosyst. Environ., 119(1-2):190-204. https://doi.org/10.1016/j.agee.2006.07.009
Chen, L., Y. Ren, Y. Zhang, J. Xu, F. Sun, Z. Zhang and Y. Wang. 2012. Genome-wide identification and expression analysis of heat-responsive and novel microRNAs in Populus tomentosa. Gene., 504(2):160-5. https://doi.org/10.1016/j.gene.2012.05.034
Colclough, M., E. Blumwald, and S.J. Colombo. 1990. The induction of heat tolerance in black spruce seedlings. In: Annual Meeting of the American Society of Plant Physiologists. Amer. Soc. Plant Physiol., 93(1):88.
Cvikrová, M., L. Gemperlová, J. Dobrá, O. Martincová, I.T. Prásil, J. Gubis and R. Vanková. 2012. Effect of heat stress on polyamine metabolism in proline-over-producing tobacco plants. Plant Sci., 182:49-58. https://doi.org/10.1016/j.plantsci.2011.01.016
Drolet, G., E.B. Dumbroff, R.L. Legge and J.E. Thompson. 1986. Radical scavenging properties of polyamines. Phytochemist, 25(2):367-71. https://doi.org/10.1016/S0031-9422(00)85482-5
Duan, J.J., J. Li, S. Guo and Y. Kang. 2008. Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J. Plant Physiol., 165(15):1620-35. https://doi.org/10.1016/j.jplph.2007.11.006
Dutta, S., S. Mohanty and B.C. Tripathy. 2009. Role of temperature stress on chloroplast biogenesis and protein import in pea. Plant Physiol., 150(2):1050-61. https://doi.org/10.1104/pp.109.137265
El-Moursi, A., K.G. El-Din and S.A. Tarraf. 2012. Physiological response of lupine plant (Lupinus termis L.) to heat hardening. Am. Eurasian J. Agric. Environ. Sci., 12(5): 660-663.
Elstner, E.F. and W. Osswald. 1994. Mechanisms of oxygen activation during plant stress. Proc. R. Soc. Edinburgh. Sect. B. Biol. Sci., 102: 131-154. https://doi.org/10.1017/S0269727000014068
Farooq, M., A. Wahid and D.J. Lee. 2009. Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol. Plant., 31(5):937-45. https://doi.org/10.1007/s11738-009-0307-2
Farooq, M., H. Bramley, J.A. Palta and K.H.M. Siddique. 2011. Heat stress in wheat during reproductive and grain-filling phases. Crit. Rev. Plant Sci., 30(6): 491-507. https://doi.org/10.1080/07352689.2011.615687
Farouk, S. 2011. Osmotic adjustment in wheat flag leaf in relation to flag leaf area and grain yield per plant. 2011. J. Stress Physiol. Biochem., 7(2): 117-138.
Feierabend, J. 1977. Capacity for chlorophyll synthesis in heat-bleached 70S ribosome-deficient rye leaves. Planta., 135(1):83-88. https://doi.org/10.1007/BF00387980
Firon, N., R. Shaked, M.M. Peet, D.M. Pharr, E. Zamski, K. Rosenfeld, L. Althan and E. Pressman. 2006. Pollen grains of heat tolerant tomato cultivars retain higher carbohydrate concentration under heat stress conditions. Sci. Hort., 109(3):212-217. https://doi.org/10.1016/j.scienta.2006.03.007
Floryszak, W.J., E. Grabikowski, J. Kubis and Z. Krzywanski. 1992. The effect of spermidine on lipid peroxidation in wheat leaves during water stress. Acta Physiol. Plant, 14(1):3-10.
Foyer, C.H. 2002. The contribution of photosynthetic oxygen metabolism to oxidative stress in plants.p.33-68. In: Inze, D. and vanMontagu, M. (ed.) Oxidative stress in plants Taylor & Francis, London.
Fu, X.Z., F. Xing, N.Q. Wang, L.Z. Peng, C.P. Chun, L. Cao, L.L. Ling and C.L. Jiang. 2014. Exogenous spermine pretreatment confers tolerance to combined high-temperature and drought stress in vitro in trifoliate orange seedlings via modulation of antioxidative capacity and expression of stress-related genes. Biotechnol. Biotechnol. Equip., 28(2):192-198. https://doi.org/10.1080/13102818.2014.909152
Geisenberg, C. and K. Stewart. 1986. Field crop management. Tomato Crop. 511-557. https://doi.org/10.1007/978-94-009-3137-4_13
Georgieva, K. 1999. Some mechanisms of damage and acclimation of the photosynthetic apparatus due to high temperature. Bulg. J. Plant Physiol., 25(3-4): 89-99.
Gosavi, G.U., A.S. Jadhav, A.A. Kale, S.R. Gadakh, B.D. Pawar and V.P. Chimote. 2014. Effect of heat stress on proline, chlorophyll content, heat shock proteins and antioxidant enzyme activity in sorghum (Sorghum bicolor) at seedlings stage. Indian J. Biotechnol., 13(3):356-363.
Goyal, M. and B. Asthir. 2010. Polyamine catabolism influences antioxidative defense mechanism in shoots and roots of five wheat genotypes under high temperature stress. Plant Growth Regul., 60(1):13-25. https://doi.org/10.1007/s10725-009-9414-8
Groppa, M.D. and M.P. Benavides. 2008. Polyamines and abiotic stress: recent advances. Amino Acids., 34(1):35-45. https://doi.org/10.1007/s00726-007-0501-8
Hall, A.E. 2000. Crop responses to environment. CRC press. https://doi.org/10.1201/9781420041088
Hanna, H.Y. and T.P. Hernandez. 1982. Response of six tomato genotypes under summer and spring weather conditions in Louisiana. Hort. Sci., 17: 758 – 759.
Hasthanasombut, S., N. Paisarnwipatpong, K. Triwitayakorn, C. Kirdmanee and K. Supaibulwatana. 2011. Expression of OsBADH1 gene in Indica rice (Oryza sativa L.) in correlation with salt, plasmolysis, temperature and light stresses. Plant Omics., 4(7):75-81. https://doi.org/10.1007/s11816-009-0123-6
Hatmi, S., C. Gruau, P. Trotel-Aziz, S. Villaume, F. Rabenoelina, F. Baillieul, P. Eullaffroy, C. Clément, A. Ferchichi and A. Aziz. 2015. Drought stress tolerance in grapevine involves activation of polyamine oxidation contributing to improved immune response and low susceptibility to Botrytis cinerea. J. Exp. Bot., 66(3):775-787. https://doi.org/10.1093/jxb/eru436
Hong, S.W. and E. Vierling. 2000. Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc. Natl. Acad. Sci., 97(4): 4392-4397. https://doi.org/10.1073/pnas.97.8.4392
Howarth, C.J. 2005. Genetic improvements of tolerance to high temperature.p. 1920.In: Ashraf, M. and Harris, P.J.C., Eds., Abiotic Stresses: Plant Resistance through Breeding and Molecular Approaches, Howarth Press Inc. New York.
Hussain, S.S., M. Ali, M. Ahmad and K.H.M. Siddique. 2011. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv., 29(3): 300-311. https://doi.org/10.1016/j.biotechadv.2011.01.003
Ibrahim, S.K. and L.A. El-Muqadam. 2019. Enhancing thermotolerance of tomato plants (Lycopersieon esculentum mill) by heat hardening of seeds. Bull. Natl. Res. Cent., 43(1):110. https://doi.org/10.1186/s42269-019-0106-x
Javanmardi, J. 2009. Scientific and applied basis for vegetable transplant production. Mashad Univ. Press, Mashad, Iran.
Javanmardi, J., M. Rahemi and M. Nasirzadeh. 2014. responses of tomato and pepper transplants to high-temperature conditioning. Int. J. Veg. Sci., 20(4): 374-391. https://doi.org/10.1080/19315260.2013.816209
Kakani, V.G., K.R. Reddy, S. Koti, T.P. Wallace, P.V.V. Prasad, V.R. Reddy and D. Zhao. 2005. Differences in in vitro pollen germination and pollen tube growth of cotton cultivars in response to high temperature. Ann. Bot., 96(1):59-67. https://doi.org/10.1093/aob/mci149
Karim, M.A., Y. Fracheboud, and P. Stamp. 1997. Heat tolerance of maize with reference of some physiological characteristics. Ann. Bangladesh Agric., 7(2): 27-33.
Khalil, S. and H.A. Moursy. 1983. Changes in some germination, morphological, physiological and reproductive characters of tomato plants as influenced by heat treatment of seed. Ann. Agric. Sci. Ain Shams Univ., Cairo., 28(3): 1099-1120.
Königshofer, H. and S. Lechner. 2002. Are polyamines involved in the synthesis of heat-shock proteins in cell suspension cultures of tobacco and alfalfa in response to high-temperature stress? Plant Physiol. Biochem., 40(1):51-9. https://doi.org/10.1016/S0981-9428(01)01347-X
Kotak, S., J. Larkindale, U. Lee, P. von Koskull-Döring, E. Vierling and K.D. Scharf. 2007. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol., 10(3): 310-316. https://doi.org/10.1016/j.pbi.2007.04.011
Krishnamurthy, R. and K.A. Bhagwat. 1989. Polyamines as modulators of salt tolerance in rice cultivars. Plant Physiol., 91(2): 500-504. https://doi.org/10.1104/pp.91.2.500
Kumari, M., R.N. Pudake, V.P. Singh and A.K. Joshi. 2013. Association of staygreen trait with canopy temperature depression and yield traits under terminal heat stress in wheat (Triticum aestivum L.). Euphytica, 190(1):87-97. https://doi.org/10.1007/s10681-012-0780-3
Larkindale, J. and E. Vierling. 2008. Core genome responses involved in acclimation to high temperature. Plant Physiol., 146(2):748-61. https://doi.org/10.1104/pp.107.112060
Li, Z., W.M. Palmer, A.P. Martin, R. Wang, F. Rainsford, Y. Jin, J.W. Patrick, Y. Yang and Y.L. Ruan. 2012. High invertase activity in tomato reproductive organs correlates with enhanced sucrose import into, and heat tolerance of, young fruit. J. Exp. Bot., 63(3):1155-66. https://doi.org/10.1093/jxb/err329
Liu, H.P., B.H. Dong, Y.Y. Zhang, Z.P. Liu and Y.L. Liu. 2004. Relationship between osmotic stress and the levels of free, conjugated and bound polyamines in leaves of wheat seedlings. Plant Sci., 166(5):1261-7. https://doi.org/10.1016/j.plantsci.2003.12.039
Liu, J.H., H. Inoue and T. Moriguchi. 2008. Salt stress-mediated changes in free polyamine titers and expression of genes responsible for polyamine biosynthesis of apple in vitro shoots. Environ. Exp. Bot., 62(1):28-35. https://doi.org/10.1016/j.envexpbot.2007.07.002
Liu, J.H., H. Kitashiba, J. Wang, Y. Ban and T. Moriguchi. 2007. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol., 24(1):117-26. https://doi.org/10.5511/plantbiotechnology.24.117
Liu, J.H., K. Nada, C. Honda, H. Kitashiba, X.P. Wen, X.M. Pang and T. Moriguchi. 2006. Polyamine biosynthesis of apple callus under salt stress: Importance of the arginine decarboxylase pathway in stress response. J. Exp. Bot., 57(11):2589-2599. https://doi.org/10.1093/jxb/erl018
Liu, J.H., W. Wang, H. Wu, X. Gong and T. Moriguchi. 2015. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci., 6:827. https://doi.org/10.3389/fpls.2015.00827
Lopes, M.S. and M.P. Reynolds. 2012. Stay-green in spring wheat can be determined by spectral reflectance measurements (normalized difference vegetation index) independently from phenology. J. Exp. Bot., 63(10):3789-3798. https://doi.org/10.1093/jxb/ers071
Min, L., Y. Li, Q. Hu, L. Zhu, W. Gao, Y. Wu, Y. Ding, S. Liu, X. Yang and X. Zhang. 2014. Sugar and auxin signaling pathways respond to high-temperature stress during anther development as revealed by transcript profiling analysis in cotton. Plant Physiol., 164(3):1293-1308. https://doi.org/10.1104/pp.113.232314
Mirzaei, M., D. Pascovici, B.J. Atwell and P.A. Haynes. 2012. Differential regulation of aquaporins, small GTPases and V-ATPases proteins in rice leaves subjected to drought stress and recovery. Proteomics, 12(6):864-877. https://doi.org/10.1002/pmic.201100389
Momcilovic, I. and Z. Ristic. 2007. Expression of chloroplast protein synthesis elongation factor, EF-Tu, in two lines of maize with contrasting tolerance to heat stress during early stages of plant development. J. Plant Physiol., 164(1): 90-9. https://doi.org/10.1016/j.jplph.2006.01.010
Morales, D., P. Rodríguez, J. Dell’Amico, E. Nicolás, A. Torrecillas and M.J. Sánchez-Blanco. 2003. High-temperature preconditioning and thermal shock imposition affects water relations, gas exchange and root hydraulic conductivity in tomato. Biol. Plant., 47(2):203. https://doi.org/10.1023/B:BIOP.0000022252.70836.fc
Moriarty, T., R. West, G. Small, D. Rao and Z. Ristic. 2002. Heterologous expression of maize chloroplast protein synthesis elongation factor (EF-Tu) enhances Escherichia coli viability under heat stress. Plant Sci., 163(6):1075-1082. https://doi.org/10.1016/S0168-9452(02)00273-X
Moschou, P.N., I.D. Delis, K.A. Paschalidis and K.A. Roubelakis-Angelakis. 2008. Transgenic tobacco plants overexpressing polyamine oxidase are not able to cope with oxidative burst generated by abiotic factors. Physiol. Plant., 133(2):140-56. https://doi.org/10.1111/j.1399-3054.2008.01049.x
Nagarajan, S., S.V.K. Jagadish, A.S.H. Prasad, A.K. Thomar, A. Anand, M. Pal and P.K. Agarwal. 2010. Local climate affects growth, yield and grain quality of aromatic and non-aromatic rice in northwestern India. Agric. Ecosyst. Environ., 138(3-4):274-81. https://doi.org/10.1016/j.agee.2010.05.012
Nayyar, H., S. Kaur, Smita, S. Kumar, K.J. Singh and K.K. Dhir. 2005. Involvement of polyamines in the contrasting sensitivity of chickpea (Cicer arietinum L.) and soybean (Glycine max (L.) Merrill.) to water deficit stress. Bot. Bull. Acad. Sin., 46(4):333-338.
Peet, M.M., D.H. Willits and R. Gardner. 1997. Response of ovule development and post-pollen production processes in male-sterile tomatoes to chronic, sub-acute high temperature stress. J. Exp. Bot., 48(1):101-11. https://doi.org/10.1093/jxb/48.1.101
Potters, G., T.P. Pasternak, Y. Guisez and M.A.K. Jansen. 2009. Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant, Cell Environ., 32(2): 158-169. https://doi.org/10.1111/j.1365-3040.2008.01908.x
Prasad, P.V.V., K.J. Boote, L.H. Allen, J.E. Sheehy and J.M.G. Thomas. 2006. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. F. Crop. Res., 95(2-3):398-411. https://doi.org/10.1016/j.fcr.2005.04.008
Qin, D., H. Wu, H. Peng, Y. Yao, Z. Ni, Z. Li, C. Zhou and Q. Sun. 2008. Heat stress-responsive transcriptome analysis in heat susceptible and tolerant wheat (Triticum aestivum L.) by using wheat genome array. BMC Genomics., 9(1):432.9. https://doi.org/10.1186/1471-2164-9-432
Qin, Y.S., S.H. Tu, W.Q. Feng and X.F. Sun. 2008. Effects of calcium, magnesium, zinc and boron fertilizers on yield and quality of field tomatoes on Chengdu Plain. Soil Fertil. Sci. China., 3: 57-59.
Queitsch, C., S.W. Hong, E. Vierling and S. Lindquist. 2000. Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell., 12(4):479-92. https://doi.org/10.2307/3871063
Rasheed, R. 2009. Salinity and extreme temperature effects on sprouting buds of sugarcane (Saccharum officinarum L.): Some histological and biochemical studies. PhD thesis, Department of Botany, University of Agriculture, Faisalabad, Pakistan.
Reda, F. and H.M.H. Mandoura. 2011. Response of enzymes activities, photosynthetic pigments, proline to low or high temperature stressed wheat plant (Triticum aestivum L.) in the presence or absence of exogenous proline or cysteine. Int. J. Acad. Res., 3(4):108-15.
Reynolds, M.P., A.J.D. Pask and D.M. Mullan. 2012. Physiological breeding I: interdisciplinary approaches to improve crop adaptation. CIMMYT.
Ristic, Z., G. Williams, G. Yang, B. Martin and S. Fullerton. 1996. Dehydration, damage to cellular membranes, and heat-shock proteins in maize hybrids from different climates. J. Plant Physiol., 149(3-4):424-32. https://doi.org/10.1016/S0176-1617(96)80144-1
Rivero, R.M., J.M. Ruiz, P.C. Garcıa, L.R. Lopez-Lefebre, E. Sánchez and L. Romero. 2001. Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci., 160(2): 315-321. https://doi.org/10.1016/S0168-9452(00)00395-2
Rossi, S., P. Burgess, D. Jespersen and B. Huang. 2017. Heat-induced leaf senescence associated with chlorophyll metabolism in bentgrass lines differing in heat tolerance. Crop Sci., 57(S1): S-169. https://doi.org/10.2135/cropsci2016.06.0542
Rudich, J., E. Zamski and Y. Regev. 1977. Genotypic variation for sensitivity to high temperature in the tomato: pollination and fruit set. Bot. Gaz., 138(4):448-452. https://doi.org/10.1086/336947
Sagor, G.H.M., T. Berberich, Y. Takahashi, M. Niitsu and T. Kusano. 2013. The polyamine spermine protects Arabidopsis from heat stress-induced damage by increasing expression of heat shock-related genes. Transgenic Res., 22(3):595-605. https://doi.org/10.1007/s11248-012-9666-3
Salin, M.L. 1988. Toxic oxygen species and protective systems of the chloroplast. Physiol. Plant., 72(3):681-689. https://doi.org/10.1111/j.1399-3054.1988.tb09182.x
Santa-Cruz, A., F. Perez-Alfocea, M. Caro and M. Acosta. 1998. Polyamines as short-term salt tolerance traits in tomato. Plant Sci., 138(1):9-16. https://doi.org/10.1016/S0168-9452(98)00143-5
Sato, S., M. Kamiyama, T. Iwata, N. Makita, H. Furukawa and H. Ikeda. 2006. Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development. Ann. Bot., 97(5): 731-738. https://doi.org/10.1093/aob/mcl037
Sato, S., M.M. Peet and J.F. Thomas. 2000. Physiological factors limit fruit set of tomato (Lycopersicon esculentum Mill.) under chronic, mild heat stress. Plant, Cell Environ., 23(7):719-726. https://doi.org/10.1046/j.1365-3040.2000.00589.x
Scafaro, A.P., P.A. Haynes and B.J. Atwell. 2010. Physiological and molecular changes in Oryza meridionalis Ng., a heat-tolerant species of wild rice. J. Exp. Bot., 61(1):191-202. https://doi.org/10.1093/jxb/erp294
Sekmen, A.H., R. Ozgur, B. Uzilday and I. Turkan. 2014. Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environ. Exp. Bot., 99:141-9. https://doi.org/10.1016/j.envexpbot.2013.11.010
Selvaraj, M. G., G. Burow., J. J. Burke, V. Belamkar, N. Puppala, and M. D. Burow. 2011. Heat stress screening of peanut (Arachis hypogaea L.) seedlings for acquired thermotolerance. Plant Growth Regulat., 65(1): 83-91. https://doi.org/10.1007/s10725-011-9577-y
Shanahan, J.F., I.B. Edwards, J.S. Quick and J.R. Fenwick. 1990. Membrane thermostability and heat tolerance of spring wheat. Crop Sci., 30(2):247-51. https://doi.org/10.2135/cropsci1990.0011183X003000020001x
Shanmugam, S., K.H. Kjaer, C.O. Ottosen, E. Rosenqvist, D. Kumari Sharma and B. Wollenweber. 2013. The alleviating effect of elevated CO2 on heat stress susceptibility of two wheat (Triticum aestivum L.) cultivars. J. Agron. Crop Sci., 199(5):340-50. https://doi.org/10.1111/jac.12023
Sharma, D. K., S. B. Andersen, C. O. Ottosen, and E. Rosenqvist, 2014: Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant., 153(2): 284–298. https://doi.org/10.1111/ppl.12245
Simões-Araújo, J.L., N.G. Rumjanek and M. Margis-Pinheiro. 2003. Small heat shock proteins genes are differentially expressed in distinct varieties of common bean. Brazilian J. Plant Physiol., 15(1):33-41. https://doi.org/10.1590/S1677-04202003000100005
Smertenko, A., P. Draber, V. Viklický and Z. Opatrný. 1997. Heat stress affects the organization of microtubules and cell division in Nicotiana tabacum cells. Plant, Cell Environ., 20(12): 1534-1542. https://doi.org/10.1046/j.1365-3040.1997.d01-44.x
Snider, J.L., D.M. Oosterhuis and E.M. Kawakami. 2010. Genotypic differences in thermotolerance are dependent upon prestress capacity for antioxidant protection of the photosynthetic apparatus in Gossypium hirsutum. Physiol. Plant., 138(3): 268-277. https://doi.org/10.1111/j.1399-3054.2009.01325.x
Snider, J.L., D.M. Oosterhuis, B.W. Skulman and E.M. Kawakami. 2009. Heat stress-induced limitations to reproductive success in Gossypium hirsutum. Physiol. Plant., 137(2):125-38. https://doi.org/10.1111/j.1399-3054.2009.01266.x
Spicher, L., G. Glauser and F. Kessler. 2016. Lipid antioxidant and galactolipid remodeling under temperature stress in tomato plants. Front. Plant Sci., 7: 167. https://doi.org/10.3389/fpls.2016.00167
Sumesh, K. V., P. Sharma-Natu and M.C. Ghildiyal. 2008. Starch synthase activity and heat shock protein in relation to thermal tolerance of developing wheat grains. Biol. Plant., 52(4): 749-753. https://doi.org/10.1007/s10535-008-0145-x
Sung, D.Y., F. Kaplan, K.J. Lee and C.L. Guy. 2003. Acquired tolerance to temperature extremes. Trends Plant Sci., 8(4):179-87. https://doi.org/10.1016/S1360-1385(03)00047-5
Suzuki N., S. Koussevitzky, R. Mittler, G. Miller. 2011. ROS and redox signaling in the response of plants to abiotic stress. Plant Cell Environ., 35(2): 259-270. https://doi.org/10.1111/j.1365-3040.2011.02336.x
Suzuki, N., S. Bajad, J. Shuman, V. Shulaev and R. Mittler. 2008. The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana. J. Biol. Chem., 283(14):9269-75. https://doi.org/10.1074/jbc.M709187200
Takahashi, S., S. Whitney, S. Itoh, T. Maruyama and M. Badger. 2008. Heat stress causes inhibition of the de novo synthesis of antenna proteins and photobleaching in cultured Symbiodinium. Proc. Natl. Acad. Sci. USA, 105(11):4203-4208. https://doi.org/10.1073/pnas.0708554105
Tewari, A.K. and B.C. Tripathy. 1998. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol., 117(3):851-8. https://doi.org/10.1104/pp.117.3.851
Tiburcio, A.F., T. Altabella, M. Bitrián and R. Alcázar. 2014. The roles of polyamines during the lifespan of plants: From development to stress. Planta., 240(1):1-8. https://doi.org/10.1007/s00425-014-2055-9
Torrigiani, P., D. Bressanin, K. Beatriz Ruiz, A. Tadiello, L. Trainotti, C. Bonghi, V. Ziosi and G. Costa. 2012. Spermidine application to young developing peach fruits leads to a slowing down of ripening by impairing ripening-related ethylene and auxin metabolism and signaling. Physiol. Plant., 146(1):86-98. https://doi.org/10.1111/j.1399-3054.2012.01612.x
Turkes, M., 2003. Sustainable technological and behavioral options for reducing of greenhouse gasemissions. In Turkish: Sera gazýsalýnýmlarýnýnazaltýlmasýiçinsürdürülebilirteknolojikvedavranýþsalseçenekler). Fifth Congress of National Environmental Engineering, Ankara, Turkey. 1:267-285.
Turner, N.C. 1981. Techniques and experimental approaches for the measurement of plant water status. Plant Soil., 58(1-3): 339-66. https://doi.org/10.1007/BF02180062
Vasseur, F., F. Pantin and D. Vile. 2011. Changes in light intensity reveal a major role for carbon balance in Arabidopsis responses to high temperature. Plant Cell Environ., 34(9):1563-76. https://doi.org/10.1111/j.1365-3040.2011.02353.x
Vierling, E. 1991. The role of heat shock proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol., 42(1): 579–620. https://doi.org/10.1146/annurev.pp.42.060191.003051
Vignjevic, M., X. Wang, J.E. Olesen and B. Wollenweber. 2015. Traits in spring wheat cultivars associated with yield loss caused by a heat stress episode after anthesis. J. Agron. Crop Sci., 201(1):32-48. https://doi.org/10.1111/jac.12085
Volkov, R.A., I.I. Panchuk, P.M. Mullineaux and F. Schöffl. 2006. Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol., 61(4): 733-746. https://doi.org/10.1007/s11103-006-0045-4
Wahid A., S. Gelani, M. Ashraf and M.R. Foolad. 2007. Heat tolerance in plants: An overview. Environ. Exp. Bot., 61(3):199-223. https://doi.org/10.1016/j.envexpbot.2007.05.011
Wahid, A. and A. Ghazanfar. 2006. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J. Plant Physiol., 163(7):723-30. https://doi.org/10.1016/j.jplph.2005.07.007
Wahid, A. and A. Shabbir. 2005. Induction of heat stress tolerance in barley seedlings by pre-sowing seed treatment with glycinebetaine. Plant Growth Regul., 46(2):133-41. https://doi.org/10.1007/s10725-005-8379-5
Wang, D. and D.S. Luthe. 2003. Heat sensitivity in a bentgrass variant. Failure to accumulate a chloroplast heat shock protein isoform implicated in heat tolerance. Plant Physiol., 133(1):319-27. https://doi.org/10.1104/pp.102.018309
Wang, J., P.P. Sun, C.L. Chen, Y. Wang, X.Z. Fu and J.H. Liu. 2011. An arginine decarboxylase gene PtADC from Poncirus trifoliata confers abiotic stress tolerance and promotes primary root growth in Arabidopsis. J. Exp. Bot., 62(8):2899-2914. https://doi.org/10.1093/jxb/erq463
Wang, R., F. Yang, X.Q. Zhang, D. Wu, C. Tan, S. Westcott, S. Broughton, C. Li, W. Zhang and Y. Xu. 2017. Characterization of a thermo-inducible chlorophyll-deficient mutant in barley. Front. Plant Sci., 8: 1936. https://doi.org/10.3389/fpls.2017.01936
Xu, S., J. Li, X. Zhang, H. Wei and L. Cui. 2006. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp. Bot., 56(3):274-85. https://doi.org/10.1016/j.envexpbot.2005.03.002
Yamasaki, S. and L. Dillenburg. 1999. Measurements of leaf relative water content in Araucaria angustifolia. Rev. Bras. Fisiol. Veg. 11(2):69-75.
Yarwood, C.E. 1961. Acquired tolerance of leaves to heat. Science, 134(3483): 941-942. https://doi.org/10.1126/science.134.3483.941
Young, L.W., R.W. Wilen and P.C. Bonham-Smith. 2004. High temperature stress of Brassica napus during flowering reduces micro-and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J. Exp. Bot., 55(396): 485-495. https://doi.org/10.1093/jxb/erh038
Zhan, J., W. Huang and L. Wang. 2003. Research of weak light stress physiology in plants. Chinese Bull. Bot., 20(01): 43–50.
Zhang, M., R. Barg, M. Yin, Y. Gueta-Dahan, A. Leikin-Frenkel, Y. Salts, S. Shabtai and G. Ben-Hayyim. 2005. Modulated fatty acid desaturation via overexpression of two distinct ω-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J., 44(3):361-71. https://doi.org/10.1111/j.1365-313X.2005.02536.x
Zhou, R., K.H. Kjær, E. Rosenqvist, X. Yu, Z. Wu and C.O. Ottosen. 2017. Physiological response to heat stress during seedling and anthesis stage in tomato genotypes differing in heat tolerance. J. Agron. Crop Sci., 203(1):68-80. https://doi.org/10.1111/jac.12166
Zhou, R., X. Yu, C.O. Ottosen, E. Rosenqvist, L. Zhao, Y. Wang, W. Yu, T. Zhao and Z. Wu. 2017. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol., 17(1):1-3. https://doi.org/10.1186/s12870-017-0974-x
Zhou, R., X. Yu, K.H. Kjær, E. Rosenqvist, C.O. Ottosen and Z. Wu. 2015. Screening and validation of tomato genotypes under heat stress using Fv/Fm to reveal the physiological mechanism of heat tolerance. Environ. Exp. Bot., 118:1-11. https://doi.org/10.1016/j.envexpbot.2015.05.006
Zivcak, M., M. Brestic, Z. Balatova, P. Drevenakova, K. Olsovska, H.M. Kalaji, X. Yang and S.I. Allakhverdiev. 2013. Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth. Res., 117(1-3): 529-46. https://doi.org/10.1007/s11120-013-9885-3
To share on other social networks, click on any share button. What are these?







