A Response-Surface Analysis of Temperature-Salinity on Growth and Survival of the Caridean Shrimp, Macrobrachium americanum (Bate, 1868)
A Response-Surface Analysis of Temperature-Salinity on Growth and Survival of the Caridean Shrimp, Macrobrachium americanum (Bate, 1868)
Fermín López-Uriostegui1, Jesus T. Ponce-Palafox2*, Fabiola Lango-Reynoso1, María R. Castañeda-Chávez1, Itzel Galaviz-Villa1, Sergio Castillo-Vargasmachuca2 and Arturo Ruiz Luna3
1Postgraduate Doctorate in Aquaculture, National Technology of Mexico /Boca del Río Institute Technology Km. 12 Road Veracruz-Córdoba. Veracruz 94290, Mexico
2Nayarit State University, Coastal Bioengineering Laboratory, Nayarit, 63000, Mexico 3Center for Research in Food and Development A.C. Mazatlan unit. Mazatlan Sinaloa, 82000, Mexico
ABSTRACT
The effect of different levels of temperature (23, 26, 29, and 32ºC) and salinity (0, 5, and 10‰) on growth performance and survival of Macrobrachium americanum was studied under controlled laboratory conditions. Sub-adult’s prawns (10.29 ± 0.27 g) were reared in these conditions for 72 days. Each experimental group contained 25 prawns in an approximately 100-L water capacity tank. The experimental design was factorial with three repetitions per treatment. Prawns were fed (commercial shrimp feed with 35% protein, 12% lipid) daily at a ratio of 10% body weight, twice a day (10:00 and 18:00 h). The optimal growth intervals were from 26 to 29°C and from 3 to 11‰ of salinity, and survival was at 23 to 29°C and 0 to 5‰. The greatest effect of the temperature-salinity interaction was on the upper extremes of response. The analysis of response surfaces showed that the final weight and weight gain increased as temperature increased and, in the upper end, salinity had a linear synergistic effect, with a greater effect on growth at high temperatures. M. americanum attains its best growth in freshwater and low salinities (< 11‰) in subtropical zones.
Article Information
Received 23 May 2018
Revised 29 July 2019
Accepted 03 May 2019
Available online 19 May 2020
Authors’ Contribution
JTPP, FLR and MRC conceived and designed the experiments. FLU and SCV performed the experiments. IGV and ARL analyzed the data. JTPP wrote the manuscript.
Key words
Caridean shrimp, Macrobrachium americanum, Specific growth rate, Optimal growth
DOI: https://dx.doi.org/10.17582/journal.pjz/20180523170525
* Corresponding author: jesus.ponce@usa.net
0030-9923/2020/0005-1903 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
INTRODUCTION
Salinity and temperature affect the physiology and, partially, the distribution and survival of aquatic organisms (Re et al., 2005). Temperature and salinity are factors that directly and indirectly influence aquatic organisms. The first factor directly controls aspects of the organism’s activity and the second indirectly influences the physiological response, such as nutrition, metabolism, growth, life cycle, and intra- and inter-specific relationships (Kinne, 1971). In brackish waters, studies of water temperature and salinity on prawns have shown that salinities above their osmotic point have a greater effect on survival than temperature (Ch and Shailender, 2013), although high mortality is registered in extreme temperatures (Aktas and Cavdar, 2012; Crisp et al., 2017). Studies of the effect of salinity and temperature on prawns of commercial interest in the world have focused mainly on M. rosenbergii in larval-postlarval production (Mohanty et al., 2016) and in juvenile stages (Habashy and Hassan, 2011; Chand et al., 2015). The separate or combined effect of these two environmental factors on growth and survival of the Macrobrachium species in Latin America has been studied in M. amazonicum (Guest and Durocher, 1979), M. holthuisi (Moreira et al., 1979), M carcinus (Choudhury, 1971) M. acanthurus (Signoret et al., 1997) and M. tenellum (Signoret et al., 1997; Vega-Villasante et al., 2011; Rodriguez-Flores et al., 2012).
In M. americanum, studies have been performed on the effect of embryological development and survival of eggs and larvae, finding that the best temperature for the development of eggs and larvae was 29°C (Sainz-Hernández et al., 2016) and a salinity of around 20‰ is required at the beginning of larval development, decreasing to around 15‰ upon reaching the postlarval stage (Holtschmit and Pfeiler, 1984). Within the models to study the individual and synergistic response of the salinity-temperature effect, the analysis of response surfaces has been used in M. rosenbergii to determine the best parameters for growth and survival with good results and a better understanding of the interaction (Agard, 1999). The aim of this study was to determine the influence of salinity and temperature on growth and survival of M. americanum sub-adults by using response-surface techniques to understand better the single and combined effect of the two factors and their influence on growth performance.
MATERIALS AND METHODS
Animal collection and experimental site
Caridean shrimp M. americanum (10.29 ± 0.27 g) were captured in the Ameca river, in the State of Nayarit, Mexico (20°53’27.00” N, 105°07’30.20” W). The study was conducted in the Coastal Bioengineering Laboratory of the National School of Fishing Engineering, in the University of the State of Nayarit, Mexico.
Experimental design
Prawns were acclimated during 2 weeks at different temperatures (23, 26, 29, and 32°C) and salinities (0, 5, and 10‰). The experiment was carried out in a cooled room, the water was maintained at 23°C and under a 12 h L:12 h D photoperiod. Temperatures above 23°C were maintained with a 300-W thermostat-controlled immersion heater, and low aeration assured mixing of the water. Prawns were stocked at 29°C and 0‰, which were increased or decreased at a rate of 3°C/h and 2 g/L/h, respectively. Seawater was obtained from an unpolluted coastal site at the beachfront and freshwater from drinking water sources (dechlorinated tap water). Temperature and salinity were controlled daily to maintain the variations within ±0.5°C and ±0.5‰, respectively, and were measured with an YSI Model 85 salinity-conductivity-temperature meter (Yellow Springs Instrument, Yellow Springs, OH, USA). Each experimental group contained 25 prawns in approximately 80-L water in a 100-L capacity tank. The experimental design was factorial with three repetitions per treatment. Prawns were fed (commercial shrimp feed with 35% protein, 12% lipid) daily at a ratio of 10% body weight, twice a day (10:00 and 18:00 h). Feed and fecal matters were removed daily. The prawns were cultured for 72 days under these conditions. The initial weight (Wi), final weight (Wf), food conversion ratio (FCR), weight gain (WG), specific growth rate (SGR), condition factor (K), and survival were calculated according to Ponce-Palafox et al. (2014).
Response-surface analysis
The influences of salinity and temperature and their combinations on growth and survival were examined using response-surface regression analysis (JMP PRO 13, 2016). To predict the optimal point (zone where SGR is maximum without having a negative effect on the prawn), a second-order polynomial function was fitted. The function correlated the relationship between independent variables and response. To correlate the two variables (temperature and salinity), the following equation was used:
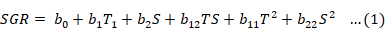
Where b0 is the model constant; T and S are independent variables, T= temperature (°C) and S= salinity (g L-1); b1 and b2 are linear coefficients (b1= linear effects of temperature and b2= linear effects of salinity); b12 is crossproduct coefficient (b12= interaction effects between temperature and salinity); and b11 and b22 are the quadratic coefficients (b11= quadratic effects of temperature and b22= quadratic effects of salinity) (Box and Youle, 1955). The software JMP Pro 13 (Version 13.2.1, created by SAS for statistical discovery, 2016, USA) was used for experimental design, data analysis, and quadratic model building. The standard response of shrimp to SGR as a result of temperature and salinity was obtained by solving the regression equation and by analyzing the response-surface contour plots using the Statistica software (Version 7, Stat Soft, Inc 2004, USA). The complete response-surface model for each response parameter was a second-order polynomial with orthogonal central composite designs.
Data analysis
Data were assessed for homogeneity of variance using Levene’s test. Analysis of variance (p < 0.05) was used to test the individual and combined effects of temperature (23, 26, 29, and 32°C) and salinity (0, 5, and 10‰) on the growth and survival parameters of M. americanum. Tukey‘s multiple comparisons test (P < 0.05) was used to compare treatments (Steel and Torrie, 1980). Before analysis, regressor variables were transformed to deviations from the mean to reduce correlation between the linear and quadratic regressors.
RESULTS
The results for weight of M. americanum at different salinities and temperatures after a period of 72 days are shown in Figure 1. Up to 10 days, there are no differences (P > 0.05) in growth in the three salinities at all temperatures tested. At 40 days, significantly (P < 0.05) lower growth was observed for prawns grown at 0‰ of salinity at 29 and 32°C. At the end of the experiment (72 days), the organisms at salinities of 5 and 10‰ grew significantly more than those at 0‰ and temperatures of 29 and 32°C. The increase in prawn weight at 29°C was the largest of all treatments.
Table I. Growth, production, and survival parameters of M. americanum at different temperatures and salinities in a recirculating system.
|
Temperature (°C) |
Salinity (‰) |
Wi (g) |
Wf (g) |
FCR |
SGR (%/day) |
K condition |
Survival (%) |
|
23 |
0 |
9.8 |
12.4c |
1.8b |
0.33d |
0.64d |
84.0a |
|
23 |
5 |
10.8 |
13.2c |
1.7b |
0.28d |
0.72c |
84.0a |
|
23 |
10 |
10.5 |
13.1c |
1.7b |
0.31d |
1.25a |
84.0a |
|
26 |
0 |
10.2 |
14.5b |
1.5a |
0.49b |
0.97a |
80.0a |
|
26 |
5 |
10.5 |
14.9b |
1.5a |
0.49b |
0.67d |
80.0b |
|
26 |
10 |
10.2 |
15.4b |
1.4a |
0.57b |
0.60d |
80.0b |
|
29 |
0 |
10.2 |
14.7b |
1.3a |
0.50b |
0.97a |
76.0b |
|
29 |
5 |
10.5 |
17.7a |
1.3a |
0.73a |
0.80b |
76.0b |
|
29 |
10 |
10.5 |
16.9a |
1.4a |
0.66a |
0.99a |
76.0b |
|
32 |
0 |
10 |
11.5d |
1.9b |
0.19e |
0.74c |
72.0b |
|
32 |
5 |
10.2 |
14.1b,c |
1.5a |
0.45c |
0.87b |
72.0b |
|
32 |
10 |
10.1 |
13.5c |
1.6a,b |
0.40c |
0.68d |
60.0c |
The maximum SGR was found at the temperature of 29°C and at the salinity of 5‰, whereas the lowest SGR was at the temperature of 32°C and salinity of 0‰ (Fig. 2). Growth parameters showed significantly (P < 0.05) that the highest final weight and SGR were determined at the temperature of 29°C and salinities of 5 and 10‰, with a low FCR (Table I). The highest K condition factor was found at 29°C and 10‰ salinity and the lowest final weight, SGR, and K condition factor were measured at 32°C and 0‰ salinity. In general, the significantly lowest survival (P < 0.05) was found with the 32°C treatment at all the salinities studied. The prawns that revealed an isometric growth (b = 3.010) were those maintained at 26°C and 10‰ of salinity (Table II). Prawns maintained in salinities of 10‰ at all temperatures tended to isometry in general terms. The weight gain declined above 32°C and was strongly reduced below 23C and above 33°C. The growth rate also decreased when salinity was below 5‰ (Fig. 3). There was a tendency to decrease the survival as salinity increased above 10‰ and temperature above 30C (Fig. 4). Constant estimates, including significance and 95% confidence intervals of the model equation, generated for final weight are presented on Table III. The linear effect of salinity and quadratic effects of temperature, significantly (P < 0.05) contributed to the variation in final weight. The linear effect of temperature and interactive effects between temperature and salinity were not significant (P > 0.05). The coefficients model was calculated on the experimental responses. The fitted model was made according to the following equation:
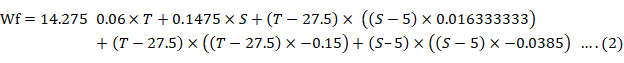
where T= temperature (°C); S= salinity (‰), and SGR= specific growth rate (%/day).
The effect of the temperature-salinity interaction on the final weight was shown in Figure 5; the plot was circular, which indicates the synergistic effect of the two factors. When temperature was in the range of 26 to 29°C and the salinity was 3 to 10.5‰, the best final weight was obtained. When salinity was maintained >10.5‰, and temperature was gradually increased from 32 to 34°C and decreased from 23 to 22°C, the final weight decreased rapidly indicating that the salinity-temperature effect is greater in the tested lower and upper ends.
Table II. Estimated parameters of the length (LT)–weight (WT) relationships for M. americanum cultured at different salinities and temperatures in a recirculating system for 72 days. Values for the slope (b) are shown.
|
Temperature (°C) |
Salinity (‰) |
a |
b |
r2 |
|
23 |
0 |
0.0048 |
3.3392 |
0.9585 |
|
23 |
5 |
0.0088 |
3.2619 |
0.9559 |
|
23 |
10 |
0.0041 |
3.6507 |
0.9609 |
|
26 |
0 |
0.0805 |
2.3127 |
0.8727 |
|
26 |
5 |
0.035 |
2.5467 |
0.9974 |
|
26 |
10 |
0.013 |
3.0108 |
0.9673 |
|
29 |
0 |
0.0083 |
3.2977 |
0.9955 |
|
29 |
5 |
0.0027 |
3.8082 |
0.9685 |
|
29 |
10 |
0.0156 |
2.9890 |
0.9964 |
|
32 |
0 |
0.0176 |
2.9564 |
0.9483 |
|
32 |
5 |
0.0045 |
3.5600 |
0.8299 |
|
32 |
10 |
0.0142 |
2.9811 |
0.9858 |
DISCUSSION
The results of this study show that juvenile and adults of M. americanum can tolerate a wide range of temperature and low salinity, although these factors will have independent and synergistic effects on growth, survival, and production, which is a similar pattern to that of several crustacean species (Ruscoe et al., 2004) found in areas adjacent to the coastal lagoon-estuarine systems of the tropical regions of the American Pacific. In general, the specific growth rate and the weight gain increased from 23 to 29°C and at 32°C began to decline in all salinities until reaching the lowest at 34°C (Fig. 3). This behavior is similar to several Macrobrachium species from tropical regions (Habshy et al., 2011). The decline in the growth of crustaceans at high temperatures has been explained because the high demand of metabolism is close to the caloric intake in these conditions, leaving minimal energy for growth (Firkins and Holdich, 1993); likewise molting frequency increases. If the osmotic gradient is also high, the prawns will use less water in the ecdysis producing a decrease in the size of the organisms, as opposed to low salinities (0 to 10‰) where there is greater availability of water with the consequent increase in size (Yen and Bart, 2008), as has been determined in the prawn M. rosenbergii (Goodwin and Hanson, 1975; Chand et al., 2015). In this latter species the osmotic point has been determined at 17 ‰ (Steel, 1980), but its best growth occurs in slightly brackish waters, around 5‰ (Nair and Salin, 2012), similar to that found in this work for M. americanum, where salinity had an effect on its culture and showed adequate results in the range of salinities from 4 to 11‰. The analysis of response surfaces showed that the final weight and weight gain increased as the temperature increased and, in the upper end, the salinity had a linear synergistic effect, having a greater effect on growth at high temperatures. The response surface approach to study the response of the physiological performance of M. americanum showed the optimum conditions of salinity-temperature for its growth and survival, and provided fundamental information for the study of the environmental physiology of this species.
In conclusion, the present study clarifies the effects of temperature and salinity and their interaction on growth and survival of M. americanum. The results confirm a relatively wider tolerance to temperature than to salinity. The greatest effect of the temperature-salinity interaction was on the upper extremes of response. The optimal growth intervals were from 26 to 29°C and from 3 to 11‰ of salinity and for survival, they were 23 to 29°C and 0 to 5‰. The information obtained provides basic data to continue
Table III. Parameter estimates from polynomial surface responses of temperature and salinity on final weight of the caridean shrimp M. americanum.
|
Parameter |
Estimate Wf (g) |
Std error |
Wald Chi- square |
Prob > Chi-Square |
95 % CI |
|
|
Low |
High |
|||||
|
Constant model |
14.275 |
2.2215463 |
41.289678 |
<0.0001* |
9.9208492 |
18.629151 |
|
Temperature |
0.06 |
0.07737 |
0.6013921 |
0.438 |
-0.091642 |
0.2116423 |
|
Salinity |
0.1475 |
0.0635659 |
5.3843774 |
0.0203* |
0.0229131 |
0.2720869 |
|
(T-27.5)*(S - 5) |
0.0163333 |
0.0189517 |
0.7427688 |
0.3888 |
-0.020811 |
0.053478 |
|
(T-27.5)*(T-27.5) |
-0.15 |
0.0288341 |
27.062645 |
<0.0001* |
-0.206514 |
-0.093486 |
|
(S - 5)*(S - 5) |
-0.0385 |
0.0220199 |
3.0569734 |
0.0804 |
-0.081658 |
0.0046582 |
|
Scale |
0.8989577 |
0.366998 |
6 |
0.0143* |
0.179655 |
1.6182605 |
with the study of the physiological response of the species under the two factors used, and to improve the implementation of management strategies in intensive and semi-intensive farming systems.
ACKNOWLEDGMENTS
The authors thank Rafael Lopez Santillan for their help in the bioassays. This study is part of F Lopez-Uriostegui PhD thesis.
Statement of conflict of interest
No potential conflict of interest was reported by the authors.
REFERENCES
Agard, J.B.R., 1999. A four-dimensional response surface analysis of the ontogeny of physiological adaptation to salinity and temperature in larvae of the palaemonid shrimp Macrobrachium rosenbergii. J. exp. Mar. Biol. Ecol., 236: 209-233. https://doi.org/10.1016/S0022-0981(98)00202-0
Aktas, M. and Cavdar, N., 2012. The combined effects of salinity and temperature on the egg hatching rate, incubation time, and survival until protozoal stages of Metapenaeus monoceros (Fabricius) (Decapoda: Penaeidae). Turk. J. Zool., 37: 249-253.
Box, G.E.P. and Youle, P.V., 1955. The exploration and exploitation of response surfaces; an example of the link between the fitted surface and the basic mechanism of the system. Biometrics, 11: 297-323. https://doi.org/10.2307/3001769
Ch, S.B. and Shailender, M., 2013. Effect of salinity and temperature on larval growth and survival of black tiger shrimp Penaeus monodon (Fabricius) in laboratory conditions. Int. J. Bio-Pharm. Res., 2: 72-77.
Chand, B.K., Trivedi, R.K., Dubey, S.K., Rout, S.K., Bega, M.M. and Das, U.K., 2015. Effect of salinity on survival and growth of giant freshwater prawn Macrobrachium rosenbergii (de Man). Aquacult. Rep., 2: 26-33. https://doi.org/10.1016/j.aqrep.2015.05.002
Choudhury, P.C., 1971. Responses of larval Macrobrachium carcinus (L.) to variations in salinity and diet (Decapoda, Palaemonidae). Crustaceana, 20: 113-120. https://doi.org/10.1163/156854069X00132
Crisp, J.A., Partridge, G.J., D’Souza, F.M.L., Tweedley, J.R., Moheimani, N.R., 2017. Effects of temperature and salinity on larval survival and development of the western school prawn Metapenaeus dalli. Int. aquat. Res., 9: 1–10. https://doi.org/10.1007/s40071-016-0151-0
Firkins, I. and Holdich, D., 1993. Thermal studies with three species of freshwater crayfish. Freshw. Crayfish, 9: 241-248.
Goodwin, H.L. and Hanson, J.A., 1975. Aquaculture of the freshwater prawn Macrobrachium species. The Oceanic Institute, Waimanalo, Hawaii. USA.
Guest, W.C. and Durocher, P.P., 1979. Palaemonid shrimp, Macrobrachium amazonicum: effects of salinity and temperature on survival. Prog. fish-cult. 41: 14-18. https://doi.org/10.1577/1548-8659(1979)41[14:PSMA]2.0.CO;2
Habashy, M.H. and Hassan, M.M.S., 2011. Effects of temperature and salinity on growth and reproduction of the freshwater prawn, Macrobrachium rosenbergii (Crustacea- Decapoda) in Egypt. Int. J. environ. Sci. Eng., 1: 83-90.
Holtschmit, K.H. and Pfeiler, E., 1984. Effect of salinity on survival and development of larvae and post-larvae of Macrobrachium americanum Bate (Decapoda, Palaemonidae). Crustaceana, 46: 23-28. https://doi.org/10.1163/156854084X00027
Kinne, O., 1971. Marine ecology: environmental factors. vol.1. Wiley, London.
Mohanty, A.K., Mohanty, S.S. and Pramanik, D.S., 2016. The combined effects of salinity and temperature on the survival of zoeae and postlarvae of Macrobrachium rosenbergii at hatchery condition in Odisha, India. Int. J. Fish. aquat. Stud. 4: 576-580.
Moreira, G.S., McNamara, J.C. and Moreira, P.S., 1979. The combined effects of temperature and salinity on the survival and moulting of early zoeae of Macrobrachium holthuisi (Decapoda: Palaemonidae). Biol. Fisiol. Anim. Univ. S. Paulo, 3: 81-93.
Nair, C.M. and Salin, K.R., 2012. Current status and prospects of farming the giant river prawn Macrobrachium rosenbergii (De Man) and the monsoon river prawn Macrobrachium malcolmsonii (H. M. Edwards) in India. Aquacul. Res., 43: 999-1014. https://doi.org/10.1111/j.1365-2109.2011.03074.x
Ponce-Palafox, J.T., López-Uriostegui, F., Arredondo-Figueroa, J.L., Benítez-Mandujano, M.A., Ulloa-Gómez, M. and Esparza-Leal, H.M. and Spanopoulos-Hernández, M., 2014. Effect of stocking size on growth performance, biomass, production, yield, and survival of caridean shrimp cage-cultured in a pond system. N. Am. J. Aquacul., 76: 430-435. https://doi.org/10.1080/15222055.2014.936538
Re, A.D., Díaz, F., Sierra, E., Rodríguez, J. and Pérez, E., 2005. Effect of salinity and temperature on thermal tolerance of brown shrimp Farfantepenaeus aztecus (Ives) (Crustacea, Penaeidae). J. Therm. Biol., 30: 618-622. https://doi.org/10.1016/j.jtherbio.2005.09.004
Rodríguez-Flores, R., Lazareno, M.M., Espinosa, L.D., Basto, M.R.R. and Vega-Villasante, F., 2012. Temperatura óptima y preferencia térmica del camarón de río Macrobrachium tenellum en la costa tropical del Pacífico Mexicano. Bol. Inst. Pesca São Paulo., 38: 121-130.
Ruscoe, I.M., Shelley, C.C. and Williams, G.R., 2004. The combined effects of temperature and salinity on growth and survival of juvenile mud crabs (Scylla serrata Forskal). Aquaculture, 238: 239-247. https://doi.org/10.1016/j.aquaculture.2004.05.030
Sainz-Hernández, J.C., Fierro-Coronado, J.A., Aguiñaga-Cruz, J.A., García-Rodríguez, L.D., Barraza-López, J.S., Santamaría-Miranda, A., Apún-Molina, J.P. and Castro-Martínez, C., 2016. Effect of temperature on the morphometric development of eggs in the prawn Macrobrachium americanum (Caridea: Palaemonidae) and larval success under experimental conditions. Invertebr. Reprod. Dev., 60: 194-200. https://doi.org/10.1080/07924259.2016.1186753
Signoret, G., Brailovsky, P. and Soto, E.G., 1997. Comportamiento osmorregulador de Macrobrachium tenellum y Macrobrachium acanthurus (Decapoda:Palaemonidae) en diferentes salinidades. Rev. Biol. Trop., 45: 1085-1091.
Steel, R.G.D. and Torrie, J.H., 1980. Principles and procedures of statistics: a biometrical approach. McGraw-Hill, New York, NY.
Vega-Villasante, F., Galavíz-Parada, J.D., Guzmán-Arroyo, M., Flores-Zepeda, C.A. and Espinosa-Chaurand, L.D., 2011. Efecto de diferentes salinidades sobre el crecimiento y supervivencia de juveniles del langostino de río Macrobrachium tenellum (Smith, 1871). Zootec. Trop., 29: 467-473.
Yen, P.T. and Bart, A.N., 2008. Salinity effects on reproduction of the giant freshwater prawn Macrobrachium rosenbergii (de Man). Aquaculture, 280: 124-128. https://doi.org/10.1016/j.aquaculture.2008.04.035
To share on other social networks, click on any share button. What are these?










